当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydrothermal Leaching Behavior of Manganese from Waste Zn–Mn Dry Batteries
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2021-02-16 , DOI: 10.1021/acssuschemeng.0c07888 Lu Zhan 1, 2 , Xiaochuan Ren 2 , Zhenming Xu 1
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2021-02-16 , DOI: 10.1021/acssuschemeng.0c07888 Lu Zhan 1, 2 , Xiaochuan Ren 2 , Zhenming Xu 1
Affiliation
|
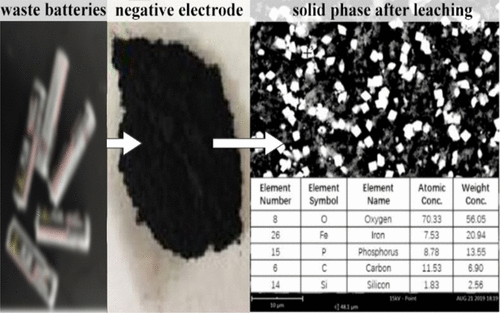
In this study, phosphoric acid and hydrogen peroxide systems were used to recover manganese from the positive electrode material of zinc–manganese dry batteries. Factors influencing leaching reaction were discussed. The results of response surface analysis showed that the degree of influence of each factor on the leaching reaction followed the trend reaction temperature > solid–liquid ratio > phosphoric acid concentration > reaction time > amount of hydrogen peroxide added. The best conditions for leaching reaction optimized through the curved surface method were as follows: 225 °C temperature, 2.28 mol/L phosphoric acid concentration, 80.9 min reaction time, 30.4 g/L solid to liquid ratio, and 3 vol % hydrogen peroxide. The average leaching efficiency of manganese under best condition was 97.3%, which was consistent with the theoretical value. This model can be used to predict the leaching efficiency of manganese. The apparent activation energy of the leaching reaction was 17.04 kJ/mol.
中文翻译:

废锌锰干电池中锰的水热浸出行为
在这项研究中,磷酸和过氧化氢系统用于从锌锰干电池的正极材料中回收锰。讨论了影响浸出反应的因素。响应面分析结果表明,各因素对浸出反应的影响程度依次为趋势反应温度>固液比>磷酸浓度>反应时间>过氧化氢的添加量。通过曲面法优化的浸出反应的最佳条件如下:温度为225°C,磷酸浓度为2.28 mol / L,反应时间为80.9 min,固液比为30.4 g / L,过氧化氢为3 vol%。最佳条件下锰的平均浸出效率为97.3%,这与理论值是一致的。该模型可用于预测锰的浸出效率。浸出反应的表观活化能为17.04kJ / mol。
更新日期:2021-03-01
中文翻译:

废锌锰干电池中锰的水热浸出行为
在这项研究中,磷酸和过氧化氢系统用于从锌锰干电池的正极材料中回收锰。讨论了影响浸出反应的因素。响应面分析结果表明,各因素对浸出反应的影响程度依次为趋势反应温度>固液比>磷酸浓度>反应时间>过氧化氢的添加量。通过曲面法优化的浸出反应的最佳条件如下:温度为225°C,磷酸浓度为2.28 mol / L,反应时间为80.9 min,固液比为30.4 g / L,过氧化氢为3 vol%。最佳条件下锰的平均浸出效率为97.3%,这与理论值是一致的。该模型可用于预测锰的浸出效率。浸出反应的表观活化能为17.04kJ / mol。

























 京公网安备 11010802027423号
京公网安备 11010802027423号