Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.4 ) Pub Date : 2021-02-13 , DOI: 10.1016/j.saa.2021.119576 Ivana N. Stojiljković , Milica P. Rančić , Aleksandar D. Marinković , Ilija N. Cvijetić , Miloš K. Milčić
|
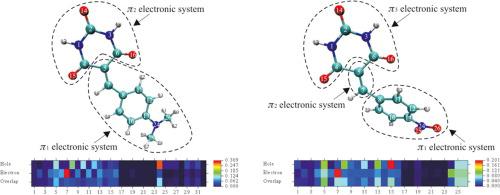
Electronic interactions in donor-π-linker-acceptor systems with barbituric acid as an electron acceptor and possible electron donor were investigated to screen promising candidates with a push–pull character based on experimental and quantum chemical studies. The tautomeric properties of 5-benzylidenebarbituric acid derivatives were studied with NMR spectra, spectrophotometric determination of the pKa values, and quantum chemical calculations. Linear solvation energy relationships (LSER) and linear free energy relationships (LFER) were applied to the spectral data - UV frequencies and 13C NMR chemical shifts. The experimental studies of the nature of the ground and excited state of investigated compounds were successfully interpreted using a computational chemistry approach including ab initio MP2 geometry optimization and time-dependent DFT calculations of excited states. Quantification of the push–pull character of barbituric acid derivatives was performed by the 13CNMR chemical shift differences, Mayer π bond order analysis, hole-electron distribution analysis, and calculations of intramolecular charge transfer (ICT) indices. The results obtained show, that when coupled with a strong electron-donor, barbituric acid can act as the electron-acceptor in push–pull systems, and when coupled with a strong electron-acceptor, barbituric acid can act as the weak electron-donor.
中文翻译:

评估对位供体和对位受体取代的5-亚苄基巴比妥酸衍生物作为推挽电子系统的潜力:实验和量子化学研究
在实验和量子化学研究的基础上,研究了以巴比妥酸为电子受体和可能的电子给体的供体-π-连接体-受体系统中的电子相互作用,以筛选具有推挽特性的有希望的候选物。用NMR光谱,分光光度法测定p K a值和量子化学计算研究了5-亚苄基巴比妥酸衍生物的互变异构性质。将线性溶剂化能量关系(LSER)和线性自由能关系(LFER)应用于光谱数据-紫外线频率和13 C NMR化学位移。使用计算化学方法成功地解释了所研究化合物的基态和激发态的实验研究从头开始进行MP2几何优化和激发态随时间的DFT计算。通过13 CNMR化学位移差异,Mayerπ键序分析,空穴电子分布分析以及分子内电荷转移(ICT)指数的计算,对巴比妥酸衍生物的推挽特性进行了定量。获得的结果表明,当与强电子给体结合时,巴比妥酸可以充当推挽系统中的电子受体;当与强电子给体结合时,巴比妥酸可以作为弱电子给体。 。

























 京公网安备 11010802027423号
京公网安备 11010802027423号