Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Carbon dioxide capture by new DBU-based DES: The relationship between ionicity and absorptive capacity
AIChE Journal ( IF 3.7 ) Pub Date : 2021-02-12 , DOI: 10.1002/aic.17244 Hui Fu 1 , Yunpeng Hou 2 , Haina Sang 1 , Tiancheng Mu 3 , Xufeng Lin 1 , Zhihua Peng 1 , Peng Li 2 , Jinhe Liu 1
AIChE Journal ( IF 3.7 ) Pub Date : 2021-02-12 , DOI: 10.1002/aic.17244 Hui Fu 1 , Yunpeng Hou 2 , Haina Sang 1 , Tiancheng Mu 3 , Xufeng Lin 1 , Zhihua Peng 1 , Peng Li 2 , Jinhe Liu 1
Affiliation
|
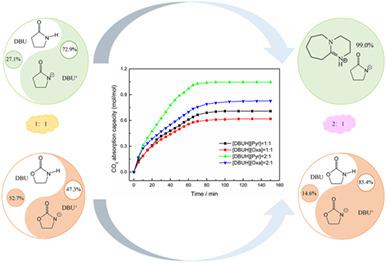
1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU)/imine, DBU/imide DESs were designed and synthesized as CO2 trapping agents. Toward this end, the optimal absorption temperature and the maximum absorption capacity were determined. It was found that ionicity is a key factor to affect the absorption capacity of CO2. Combined with the ionicity calculation, a new CO2 capture mechanism has been proposed. HBD in DES can be divided into two parts. One can undergo proton transfer, obeying anion 2:1 absorption, whereas the other, without proton transfer, is amine 1:1 absorption. The degree of proton transfer in DES determines the ionicity and affects the absorption performance. The DFT calculation results confirmed this mechanism by micro perspective. DBU/2-Pyrrolidinone and DBU/Oxazolidinone were proved to be good CO2 trapping agents with high absorption capacity, being 1.064 mol CO2/mol DES and 0.827 mol CO2/mol DES, respectively, and excellent recyclability when the mole ratio of HBA:HBD is 2:1.
中文翻译:

新的基于 DBU 的 DES 捕获二氧化碳:离子性和吸收能力之间的关系
1,8-二氮杂双环[5.4.0] undec-7-ene (DBU)/亚胺、DBU/酰亚胺DESs被设计和合成为CO 2捕集剂。为此,确定了最佳吸收温度和最大吸收能力。发现离子性是影响CO 2吸收能力的关键因素。结合离子度计算,新的 CO 2提出了捕获机制。DES 中的 HBD 可分为两部分。一种可以进行质子转移,服从阴离子 2:1 吸收,而另一种没有质子转移,是胺 1:1 吸收。DES中质子转移的程度决定了离子性并影响吸收性能。DFT 计算结果从微观角度证实了这种机制。DBU/2-吡咯烷酮和DBU/恶唑烷酮被证明是良好的CO 2捕集剂,具有高吸收能力,分别为1.064 mol CO 2 /mol DES和0.827 mol CO 2 /mol DES,当摩尔比为HBA:HBD 为 2:1。
更新日期:2021-02-12
中文翻译:

新的基于 DBU 的 DES 捕获二氧化碳:离子性和吸收能力之间的关系
1,8-二氮杂双环[5.4.0] undec-7-ene (DBU)/亚胺、DBU/酰亚胺DESs被设计和合成为CO 2捕集剂。为此,确定了最佳吸收温度和最大吸收能力。发现离子性是影响CO 2吸收能力的关键因素。结合离子度计算,新的 CO 2提出了捕获机制。DES 中的 HBD 可分为两部分。一种可以进行质子转移,服从阴离子 2:1 吸收,而另一种没有质子转移,是胺 1:1 吸收。DES中质子转移的程度决定了离子性并影响吸收性能。DFT 计算结果从微观角度证实了这种机制。DBU/2-吡咯烷酮和DBU/恶唑烷酮被证明是良好的CO 2捕集剂,具有高吸收能力,分别为1.064 mol CO 2 /mol DES和0.827 mol CO 2 /mol DES,当摩尔比为HBA:HBD 为 2:1。


























 京公网安备 11010802027423号
京公网安备 11010802027423号