Journal of Rare Earths ( IF 4.9 ) Pub Date : 2021-02-12 , DOI: 10.1016/j.jre.2021.02.003 Wenhu Luo 1, 2 , Qingjun Chen 1, 2 , Li Ji 1 , Xinyuan Peng 1 , Guosheng Huang 2
|
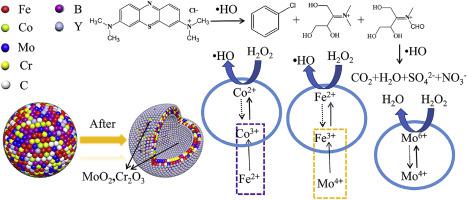
Metallic glasses have recently attracted great attention in terms of degrading dyes and other organic pollutants as an environmentally friendly material for wastewater remediation. Herein, we report a new type of amorphous catalyst Fe41Co7Cr15Mo14C15B6Y2 hollow balls. Results demonstrate that the catalyst can still completely decolorize the 20 mg/L methylene blue (MB) solution after reused for 50 times under conditions of pH = 5, catalyst content 0.5 g/L, and temperature 80 °C. The catalyst is easily broken during degradation, so the inner surface also provides additional active sites. The Fe41Co7Cr15Mo14C15B6Y2 amorphous alloy hollow balls were characterized by energy dispersive X-ray specroscopy (EDS), scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy (XPS), respectively. The elements in the catalytic system have a synergistic catalytic effect. Redox cycle Fe2+/Fe3+, Co2+/Co3+ and Mo4+/Mo6+ promote mutual conversion and accelerate the catalytic process of their reaction with H2O2, forming a self-stable redox cycle process. Among them, Fe2+ promotes the conversion of Co3+ to Co2+, and Mo4+ promotes the conversion of Fe3+ to Fe2+, mainly Fe2+ and Co2+ react with H2O2 to generate •OH. Mo and Cr elements form MoO2 and Cr2O3 plasma compounds on the surface, which act as a protective film to make the catalyst more stable and be repeated used more frequently.
中文翻译:

Fe41Co7Cr15Mo14C15B6Y2非晶合金空心球中各种元素对亚甲基蓝催化降解的协同作用
金属玻璃最近在降解染料和其他有机污染物方面引起了极大的关注,作为一种用于废水修复的环保材料。在此,我们报道了一种新型非晶催化剂Fe 41 Co 7 Cr 15 Mo 14 C 15 B 6 Y 2空心球。结果表明,在pH = 5、催化剂含量0.5 g/L、温度80 ℃的条件下,该催化剂重复使用50次后仍能完全脱色20 mg/L亚甲蓝(MB)溶液。催化剂在降解过程中很容易被破坏,因此内表面也提供了额外的活性位点。Fe 41 Co 7 Cr15 Mo 14 C 15 B 6 Y 2非晶合金空心球分别通过能量色散X射线光谱(EDS)、扫描电子显微镜(SEM)和X射线光电子能谱(XPS)表征。催化体系中的元素具有协同催化作用。氧化还原循环Fe 2+ /Fe 3+、Co 2+ /Co 3+和Mo 4+ /Mo 6+促进相互转化,加速它们与H 2 O 2反应的催化过程,形成自稳定的氧化还原循环过程. 其中,Fe 2+促进Co 3+向Co 2+的转化,Mo 4+促进Fe 3+向Fe 2+的转化,主要是Fe 2+和Co 2+与H 2 O 2反应生成•OH。Mo和Cr元素在表面形成MoO 2和Cr 2 O 3等离子化合物,起到保护膜的作用,使催化剂更稳定,重复使用更频繁。

























 京公网安备 11010802027423号
京公网安备 11010802027423号