当前位置:
X-MOL 学术
›
Chem. Rec.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development of Dipolarophiles for Catalytic Asymmetric Cycloadditions through Pd-π-Allyl Zwitterions
The Chemical Record ( IF 6.6 ) Pub Date : 2021-02-11 , DOI: 10.1002/tcr.202000177 Cun Yang 1 , Zhi-Xiong Yang 1 , Chang-Hua Ding 1 , Bin Xu 1 , Xue-Long Hou 2
The Chemical Record ( IF 6.6 ) Pub Date : 2021-02-11 , DOI: 10.1002/tcr.202000177 Cun Yang 1 , Zhi-Xiong Yang 1 , Chang-Hua Ding 1 , Bin Xu 1 , Xue-Long Hou 2
Affiliation
|
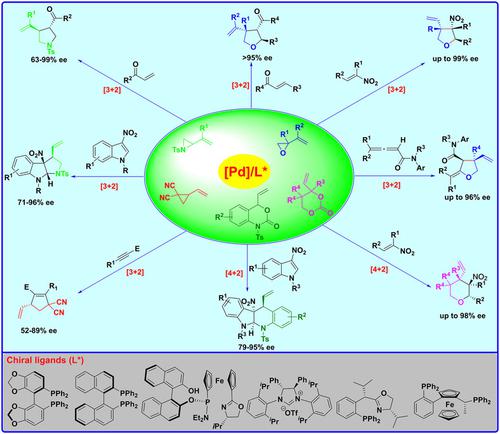
The development of new and efficient methodology for the construction of optically active molecules is of great interest in both synthetic organic and medicinal chemistry fields. To this end, the personal account summarizes our studies on the development of electron-deficient alkenes, allenes, and alkynes containing single activator as new dipolarophiles for Pd-catalyzed asymmetric cycloaddition reactions. These new dipolarophiles can participate in Pd-catalyzed asymmetric [3+2] and [4+2] cycloadditions through Pd-π-allyl 1,3- and 1,4-zwitterions in-situ generated by the reaction of Pd(0) catalyst with vinyl aziridines, vinyl epoxides, vinyl cyclopropanes, 4-vinyl-1,3-dioxan-2-ones, and vinyl benzoxazinanones. These [3+2] and [4+2] cycloadditions provide efficient approaches to a wide range of enantiomerically enriched five- and six-membered ring compounds containing contiguous chiral centers with high to excellent chemo-, diastereo-, and enantioselectivities. The utilities of these protocols are demonstrated by transformation of the cycloadducts into other useful chiral building blocks. DFT calculations reveal the dissimilar reactivity of different electron deficient alkenes and rationalize the mechanism and stereo-control of the reaction. A Pd-catalyzed inverse [3+2] cycloaddition is disclosed.
中文翻译:

通过 Pd-π-烯丙基两性离子催化不对称环加成反应的亲偶极试剂的开发
用于构建光学活性分子的新型高效方法的开发在合成有机和药物化学领域都具有重要意义。为此,个人帐户总结了我们在开发含有单一活化剂的缺电子烯烃、丙二烯和炔烃作为 Pd 催化的不对称环加成反应的新偶极体的研究。这些新的偶极体可以通过 Pd(0) 反应产生的原位 Pd-π-烯丙基 1,3- 和 1,4-两性离子参与 Pd 催化的不对称 [3+2] 和 [4+2] 环加成反应具有乙烯基氮丙啶、乙烯基环氧化物、乙烯基环丙烷、4-乙烯基-1,3-二恶烷-2-酮和乙烯基苯并恶嗪酮的催化剂。这些 [3+2] 和 [4+2] 环加成为各种富含对映异构体的五元和六元环化合物提供了有效的方法,这些化合物含有连续的手性中心,具有高至极好的化学选择性、非对映选择性和对映选择性。这些协议的效用通过将环加合物转化为其他有用的手性构件来证明。DFT 计算揭示了不同缺电子烯烃的不同反应性,并使反应的机理和立体控制合理化。公开了Pd催化的逆[3+2]环加成。DFT 计算揭示了不同缺电子烯烃的不同反应性,并使反应的机理和立体控制合理化。公开了Pd催化的逆[3+2]环加成。DFT 计算揭示了不同缺电子烯烃的不同反应性,并使反应的机理和立体控制合理化。公开了Pd催化的逆[3+2]环加成。
更新日期:2021-02-11
中文翻译:

通过 Pd-π-烯丙基两性离子催化不对称环加成反应的亲偶极试剂的开发
用于构建光学活性分子的新型高效方法的开发在合成有机和药物化学领域都具有重要意义。为此,个人帐户总结了我们在开发含有单一活化剂的缺电子烯烃、丙二烯和炔烃作为 Pd 催化的不对称环加成反应的新偶极体的研究。这些新的偶极体可以通过 Pd(0) 反应产生的原位 Pd-π-烯丙基 1,3- 和 1,4-两性离子参与 Pd 催化的不对称 [3+2] 和 [4+2] 环加成反应具有乙烯基氮丙啶、乙烯基环氧化物、乙烯基环丙烷、4-乙烯基-1,3-二恶烷-2-酮和乙烯基苯并恶嗪酮的催化剂。这些 [3+2] 和 [4+2] 环加成为各种富含对映异构体的五元和六元环化合物提供了有效的方法,这些化合物含有连续的手性中心,具有高至极好的化学选择性、非对映选择性和对映选择性。这些协议的效用通过将环加合物转化为其他有用的手性构件来证明。DFT 计算揭示了不同缺电子烯烃的不同反应性,并使反应的机理和立体控制合理化。公开了Pd催化的逆[3+2]环加成。DFT 计算揭示了不同缺电子烯烃的不同反应性,并使反应的机理和立体控制合理化。公开了Pd催化的逆[3+2]环加成。DFT 计算揭示了不同缺电子烯烃的不同反应性,并使反应的机理和立体控制合理化。公开了Pd催化的逆[3+2]环加成。


























 京公网安备 11010802027423号
京公网安备 11010802027423号