Vibrational Spectroscopy ( IF 2.5 ) Pub Date : 2021-02-10 , DOI: 10.1016/j.vibspec.2021.103229 Aravindhanathan Venkatesan , Arun Radhakrishnan , Gowthamarajan Kuppuswamy , Sachin Kumar Singh
|
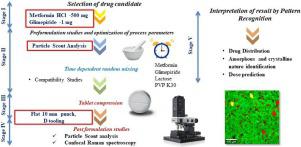
Low dose mixing and its optimization process parameters are facing more challenges. Moreover, minor variation in a dose will tend to significantly have a huge impact on the products’ performance including safety and efficacy of the dosage form. Hence, the characterization in every stage of product development of a low dose compound is mandatory. For this purpose, glimepiride and metformin in the ratio of 1:500 was formulated into tablet deciphering a fixed-dose combination product. The tablet was prepared by wet granulation method and optimized by time-dependent random mixing. Confocal Raman microscopy was employed for the present work for evaluation of compatibility studies, in-process and post-compression parameters. Further, the microscopical evaluation was done for the particle scout analysis. The obtained results indicated that the powder blend was compatible and provided uniform mixing in 20 minutes. Microscopical evaluation has shown that glimepiride still existed in the amorphous state even after compression. Moreover, the glimepiride area in the single-layer was found to be 0.06%. With this, we propose that confocal Raman microscopy plays the promising role in the detectability of the glimepiride. Therefore, in future, the layer by layer analysing can be opted to study the entire spatial arrangement of the API and excipients, which may tend to have a better understanding of dissolution and their related parameters.
中文翻译:

使用拉曼成像技术检测固定剂量组合中小剂量格列美脲的可检测性和定量
低剂量混合及其优化工艺参数面临更多挑战。而且,剂量的微小变化将趋向于对产品性能产生重大影响,包括剂型的安全性和有效性。因此,低剂量化合物在产品开发的每个阶段都必须进行表征。为此目的,将格列美脲和二甲双胍以1:500的比例配制成可破译固定剂量组合产品的片剂。通过湿法制粒制备片剂,并通过时间依赖性随机混合对其进行优化。共焦拉曼显微镜用于目前的工作,以评估相容性研究,过程中和压缩后的参数。此外,对微粒侦察分析进行了显微镜评估。所得结果表明该粉末共混物是相容的并且在20分钟内提供了均匀的混合。显微镜评估显示格列美脲即使在压缩后仍以非晶态存在。另外,发现单层的格列美脲面积为0.06%。因此,我们建议共聚焦拉曼显微镜在格列美脲的可检测性中起有希望的作用。因此,将来可以选择逐层分析来研究API和赋形剂的整个空间排列,这可能会更好地理解溶出度及其相关参数。发现单层中的格列美脲面积为0.06%。因此,我们建议共焦拉曼显微镜在格列美脲的可检测性中发挥有希望的作用。因此,将来可以选择逐层分析来研究API和赋形剂的整个空间排列,这可能会更好地理解溶出度及其相关参数。发现单层中的格列美脲面积为0.06%。因此,我们建议共聚焦拉曼显微镜在格列美脲的可检测性中起有希望的作用。因此,将来可以选择逐层分析来研究API和赋形剂的整个空间排列,这可能会更好地理解溶出度及其相关参数。


























 京公网安备 11010802027423号
京公网安备 11010802027423号