当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Experiments and DFT Computations Combine to Decipher Fe-Catalyzed Amidine Synthesis through Nitrene Transfer and Nitrile Insertion
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-02-05 , DOI: 10.1021/acscatal.0c03791 Guillaume Coin 1, 2 , Patrick Dubourdeaux 1 , Frédéric Avenier 3 , Ranjan Patra 1, 4 , Ludovic Castro 5 , Colette Lebrun 5 , Pierre-Alain Bayle 6 , Jacques Pécaut 6 , Geneviève Blondin 1 , Pascale Maldivi 5 , Jean-Marc Latour 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-02-05 , DOI: 10.1021/acscatal.0c03791 Guillaume Coin 1, 2 , Patrick Dubourdeaux 1 , Frédéric Avenier 3 , Ranjan Patra 1, 4 , Ludovic Castro 5 , Colette Lebrun 5 , Pierre-Alain Bayle 6 , Jacques Pécaut 6 , Geneviève Blondin 1 , Pascale Maldivi 5 , Jean-Marc Latour 1
Affiliation
|
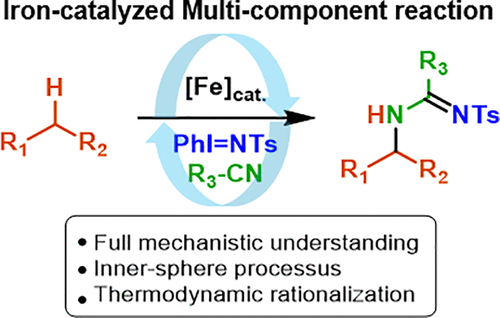
Multicomponent reactions are attracting strong interest as they contribute to the development of more efficient synthetic chemistry. Understanding their mechanism is thus an important issue to optimize their operation. However, it is also a challenging task owing to the complexity of the succession of molecular events involved. Computational methods have recently proven to be of utmost interest to help decipher some of these processes, and the development of integrated experimental and theoretical approaches thus appears as the most powerful means to understand these mechanisms at the molecular level. A good example is given by the synthesis of amidines which are important pharmaceutical compounds. Their synthesis requires the association of three components, often an alkyne, a secondary amine, and an organic azide as the nitrene precursor. We found that an alternative way is offered by an Fe-catalyzed combination of a hydrocarbon, a nitrile, and a nitrene which gives amidines in good yields under mild conditions. The efficiency of the transformation and the paucity of mechanistic information on these reactions prompted us to thoroughly investigate its mechanism. Several mechanistic scenarios were explored using experimental techniques, including radical trap and 15N labeling studies, combined with density-functional theory (DFT) calculations of reaction profiles. This allowed us to show that the amidination reaction involves the trapping of an intermediate substrate cation by an Fe-released acetonitrile molecule pointing to a true multicomponent reaction occurring exclusively within the cage around the metal center. Moreover, the calculated energy barriers of the individual steps explained how amidination outweighs direct amination in these reactions. The perfect consistency between DFT results and specific experiments to validate them strongly supports these mechanistic conclusions and highlights the potency of this combined approach.
中文翻译:

实验和DFT计算相结合,通过硝基转移和腈插入来解密铁催化的idine合成
多组分反应吸引了人们的极大兴趣,因为它们有助于开发更有效的合成化学。因此,了解它们的机制是优化其操作的重要问题。然而,由于所涉及的分子事件的连续性的复杂性,这也是一项具有挑战性的任务。最近,计算方法被证明对帮助解密其中的某些过程极为重要,因此,综合实验和理论方法的发展似乎是在分子水平上理解这些机理的最有力手段。are的合成是一个很好的例子,which是重要的药物化合物。它们的合成需要三组分的结合,通常是炔烃,仲胺和有机叠氮化物作为腈前体。我们发现,铁催化的烃,腈和腈的组合提供了另一种方法,该组合在温和的条件下以高收率得到am。转化的效率以及有关这些反应的机械信息不足,促使我们彻底研究其机理。使用实验技术探索了多种机械情景,包括自由基陷阱和15 N标记研究,结合反应谱的密度泛函理论(DFT)计算。这使我们能够证明酰胺化反应涉及通过铁释放的乙腈分子捕获中间基质阳离子,这表明真正的多组分反应只发生在金属中心周围的笼子中。而且,计算出的各个步骤的能垒解释了酰胺化在这些反应中如何胜过直接胺化。DFT结果与验证它们的特定实验之间的完美一致性有力地支持了这些机理结论,并突出了这种组合方法的潜力。
更新日期:2021-02-19
中文翻译:

实验和DFT计算相结合,通过硝基转移和腈插入来解密铁催化的idine合成
多组分反应吸引了人们的极大兴趣,因为它们有助于开发更有效的合成化学。因此,了解它们的机制是优化其操作的重要问题。然而,由于所涉及的分子事件的连续性的复杂性,这也是一项具有挑战性的任务。最近,计算方法被证明对帮助解密其中的某些过程极为重要,因此,综合实验和理论方法的发展似乎是在分子水平上理解这些机理的最有力手段。are的合成是一个很好的例子,which是重要的药物化合物。它们的合成需要三组分的结合,通常是炔烃,仲胺和有机叠氮化物作为腈前体。我们发现,铁催化的烃,腈和腈的组合提供了另一种方法,该组合在温和的条件下以高收率得到am。转化的效率以及有关这些反应的机械信息不足,促使我们彻底研究其机理。使用实验技术探索了多种机械情景,包括自由基陷阱和15 N标记研究,结合反应谱的密度泛函理论(DFT)计算。这使我们能够证明酰胺化反应涉及通过铁释放的乙腈分子捕获中间基质阳离子,这表明真正的多组分反应只发生在金属中心周围的笼子中。而且,计算出的各个步骤的能垒解释了酰胺化在这些反应中如何胜过直接胺化。DFT结果与验证它们的特定实验之间的完美一致性有力地支持了这些机理结论,并突出了这种组合方法的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号