当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chiral Allylic Amine Synthesis Enabled by the Enantioselective CpXRh(III)-Catalyzed Carboaminations of 1,3-Dienes
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-02-05 , DOI: 10.1021/acscatal.0c04777 Liexin Wu 1 , Huiying Xu 1 , Hui Gao 1 , Liping Li 1 , Weijie Chen 1 , Zhi Zhou 1 , Wei Yi 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-02-05 , DOI: 10.1021/acscatal.0c04777 Liexin Wu 1 , Huiying Xu 1 , Hui Gao 1 , Liping Li 1 , Weijie Chen 1 , Zhi Zhou 1 , Wei Yi 1
Affiliation
|
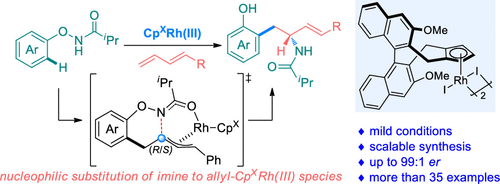
Considering the importance of the chiral allylic amine structural motif and the rarity of synthetic methods toward their construction, herein, we report a CpXRh(III)-catalyzed enantioselective intermolecular carboamination of 1,3-dienes via N-phenoxy amides-derived intermolecular aryl C–H activation and intramolecular amide transfer. The methodology enables the direct synthesis of a variety of chiral allylic amines with the embedment of phenol functionalities and proceeds under mild conditions with sequential formation of a completely regioselective C–C bond and a highly enantioselective C–N bond. Integrated experimental and computational mechanistic studies reveal an unusual Rh(III)–Rh(I)–Rh(III) catalytic pathway, in which an alkene insertion/π-allylation/intramolecular nucleophilic substitution cascade was involved for this transformation. Besides, synthetic application in the derivation of natural products and the late-stage assembly of bioactive complexes has also been demonstrated, which further strengthens the synthetic utility of this approach.
中文翻译:

对映选择性Cp X Rh(III)催化的1,3-二烯碳氨基化手性烯丙基胺的合成。
考虑到手性烯丙基胺结构基序的重要性以及合成方法对其构建的稀有性,在此,我们报道了Cp X Rh(III)催化的N对1,3-二烯的对映选择性分子间碳氨基化反应-苯氧基酰胺衍生的分子间芳基C–H活化和分子内酰胺转移。该方法可以嵌入酚官能团,直接合成多种手性烯丙基胺,并在温和条件下进行,形成顺序完整的区域选择性C–C键和高度对映选择性C–N键。综合的实验和计算机理研究揭示了一种不寻常的Rh(III)–Rh(I)–Rh(III)催化途径,其中烯烃插入/π-烯丙基化/分子内亲核取代级联参与该转化。此外,还证明了在天然产物的衍生和生物活性复合物的后期组装中的合成应用,这进一步增强了该方法的合成效用。
更新日期:2021-02-19
中文翻译:

对映选择性Cp X Rh(III)催化的1,3-二烯碳氨基化手性烯丙基胺的合成。
考虑到手性烯丙基胺结构基序的重要性以及合成方法对其构建的稀有性,在此,我们报道了Cp X Rh(III)催化的N对1,3-二烯的对映选择性分子间碳氨基化反应-苯氧基酰胺衍生的分子间芳基C–H活化和分子内酰胺转移。该方法可以嵌入酚官能团,直接合成多种手性烯丙基胺,并在温和条件下进行,形成顺序完整的区域选择性C–C键和高度对映选择性C–N键。综合的实验和计算机理研究揭示了一种不寻常的Rh(III)–Rh(I)–Rh(III)催化途径,其中烯烃插入/π-烯丙基化/分子内亲核取代级联参与该转化。此外,还证明了在天然产物的衍生和生物活性复合物的后期组装中的合成应用,这进一步增强了该方法的合成效用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号