Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.4 ) Pub Date : 2021-02-05 , DOI: 10.1016/j.saa.2021.119550 Pujarini Banerjee , Prasenjit Pandey , Biman Bandyopadhyay
|
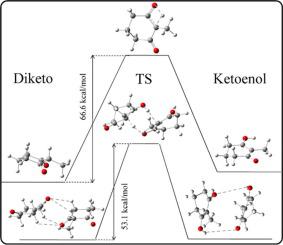
Molecular association and its impact on the keto-enol tautomerization of 2-methyl-1,3-cyclohexanedione (MCHD) have been investigated in low temperature argon matrix and thin solid film. The system exists exclusively in diketo tautomeric form in argon matrix. The C H⋯O H-bonded homodimers of the diketo tautomer are produced by annealing the matrix at 28 K. No trace of the keto-enol tautomer is observed in matrix isolated homodimers in the temperature range of 8–28 K. However, tautomeric conversion initiates in a thin film of pure diketo tautomer when the temperature of the film is raised to ~170 K. Transition state calculations on the monomeric and dimeric MCHD demonstrate that C
H⋯O H-bonded homodimers of the diketo tautomer are produced by annealing the matrix at 28 K. No trace of the keto-enol tautomer is observed in matrix isolated homodimers in the temperature range of 8–28 K. However, tautomeric conversion initiates in a thin film of pure diketo tautomer when the temperature of the film is raised to ~170 K. Transition state calculations on the monomeric and dimeric MCHD demonstrate that C H⋯O H-bond formations between diketo tautomers play a vital role in lowering the tautomerization barrier. However, the extent of C
H⋯O H-bond formations between diketo tautomers play a vital role in lowering the tautomerization barrier. However, the extent of C H⋯O H-bonded dimer formation in matrix isolation, as well as extent of tautomerization in the neat sample are found to be smaller than that for the previously reported 1,3-cyclohexanedione (CHD) under similar experimental conditions (J. Phys. Chem. A 2012, 116, 3836–3845). Electronic structure calculations suggest that formation of the C
H⋯O H-bonded dimer formation in matrix isolation, as well as extent of tautomerization in the neat sample are found to be smaller than that for the previously reported 1,3-cyclohexanedione (CHD) under similar experimental conditions (J. Phys. Chem. A 2012, 116, 3836–3845). Electronic structure calculations suggest that formation of the C H⋯O H-bonded dimer is less feasible in presence of the bulky 2-methyl groups of MCHD, as compared to CHD. Additionally, the transition state geometry of the dimeric keto-enol product of MCHD, as compared to the same for CHD, is more strained and offers a weaker C
H⋯O H-bonded dimer is less feasible in presence of the bulky 2-methyl groups of MCHD, as compared to CHD. Additionally, the transition state geometry of the dimeric keto-enol product of MCHD, as compared to the same for CHD, is more strained and offers a weaker C H---O H-bond that contributes to lesser tautomeric conversion in the former.
H---O H-bond that contributes to lesser tautomeric conversion in the former.
中文翻译:

CH⋯O H键介导的2-甲基-1,3-环己二酮互变异构:红外光谱和理论研究的结合
在低温氩气基质和固体薄膜中,研究了分子缔合及其对2-甲基-1,3-环己二酮(MCHD)的酮-烯醇互变异构的影响。该系统仅以二酮互变异构形式存在于氩气基质中。 二酮互变异构体的C H⋯O H键合同二聚体是通过在28 K的温度下对基质进行退火而制得的。在8至28 K的温度范围内,在基质分离的同二聚体中未观察到任何酮-烯醇互变异构体。当薄膜的温度升至约170 K时,转化将在纯的二酮互变异构体薄膜中引发。对单体和二聚体MCHD的过渡态计算表明,
二酮互变异构体的C H⋯O H键合同二聚体是通过在28 K的温度下对基质进行退火而制得的。在8至28 K的温度范围内,在基质分离的同二聚体中未观察到任何酮-烯醇互变异构体。当薄膜的温度升至约170 K时,转化将在纯的二酮互变异构体薄膜中引发。对单体和二聚体MCHD的过渡态计算表明, 二酮互变异构体之间的C H⋯O H键形成在其中起着至关重要的作用。降低互变异构障碍。但是,C的程度
二酮互变异构体之间的C H⋯O H键形成在其中起着至关重要的作用。降低互变异构障碍。但是,C的程度 在类似的实验条件下,发现基质分离中H = O H键合的二聚体形成以及纯样品中的互变异构程度要小于先前报道的1,3-环己二酮(CHD)(J.化学式甲2012,116,3836-3845)。电子结构计算表明,形成的C-
在类似的实验条件下,发现基质分离中H = O H键合的二聚体形成以及纯样品中的互变异构程度要小于先前报道的1,3-环己二酮(CHD)(J.化学式甲2012,116,3836-3845)。电子结构计算表明,形成的C-  ħ⋯ö氢键二聚体中的庞大的2-甲基存在不太可行小号MCHD的,相比于CHD。另外,与CHD相比,MCHD的二聚酮-烯醇产物的过渡态几何形状更易变形,并提供较弱的C
ħ⋯ö氢键二聚体中的庞大的2-甲基存在不太可行小号MCHD的,相比于CHD。另外,与CHD相比,MCHD的二聚酮-烯醇产物的过渡态几何形状更易变形,并提供较弱的C  H-- O H键,从而有助于降低前者的互变异构转化率。
H-- O H键,从而有助于降低前者的互变异构转化率。



























 京公网安备 11010802027423号
京公网安备 11010802027423号