Journal of Rare Earths ( IF 4.9 ) Pub Date : 2021-02-05 , DOI: 10.1016/j.jre.2021.01.019 Melissa Fairley , M.M. Varuni S. Livera , Wanmin Chen , Jorge H.S.K. Monteiro , Adam Schmitt , Ana de Bettencourt-Dias , Sue Roberts , Zhiping Zheng
|
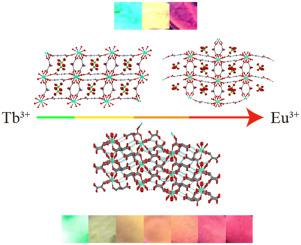
Two series of lanthanide-containing metal–organic frameworks (Ln-MOFs) of the general formula {[Ln(HIDA)2H2O]ClO4·H2O}n (Ln = La (1), Nd (2), Eu (3), Gd (4), Tb (5), Eu:Tb (6); H2IDA = iminodiacetic acid) and [Ln(TT) (HTT) (H2O)3]n (Ln = Eu (7), Gd (8), Tb (9), Dy (10), and Eu:Tb (11); H2TT = tartaric acid) were synthesized by reacting Ln(ClO4)3 with iminodiacetic acid and L-tartaric acid, respectively. All compounds were structurally characterized by single-crystal X-ray diffraction. Elemental analyses are consistent with the corresponding crystallographically generated formulas. Moreover, the luminescence properties of both the single and mixed-lanthanide complexes were studied. Near infrared, red, and green emissions that are characteristic of Nd(III), Eu(III), and Tb(III) are observed for 2, 3/7, and 5/9, respectively. For the two mixed-lanthanide complex systems 6 and 11, depending on the relative amount of Eu(III) and Tb(III), the color of emission can be fine-tuned. It is found that a small amount of Eu(III) is adequate for the observation of the most intense transition of Eu(III). This is believed to be a result of energy transfer from Tb(III) to Eu(III) within the same complex - a conclusion supported by the significantly shortened lifetime of Tb(III) and the accompanying enhanced lifetime of Eu(III) in the mixed-lanthanide complex with respect to the corresponding values for the pure Tb(III) and Eu(III) complexes with the same ligand.
中文翻译:

镧系亚氨基二乙酸盐和酒石酸盐的金属有机骨架:合成,结构表征和发光性质-纪念徐光宪院士诞辰100周年
通式为{[Ln(HIDA)2 H 2 O] ClO 4 ·H 2 O} n(Ln = La(1),Nd(2)的两个系列的含镧系元素的金属有机骨架(Ln-MOFs ) ,Eu(3),Gd(4),Tb(5),Eu:Tb(6); H 2 IDA =亚氨基二乙酸)和[Ln(TT)(HTT)(H 2 O)3 ] n(Ln = Eu(7),Gd(8),Tb(9),Dy(10)和Eu:Tb(11); H 2分别通过使Ln(ClO 4)3与亚氨基二乙酸和L-酒石酸反应合成TT(酒石酸)。所有化合物的结构均通过单晶X射线衍射表征。元素分析与相应的晶体学生成的公式一致。此外,还研究了镧系元素和复合镧系元素的发光特性。近红外,红,观察到绿色的排放是钕(III),铕(III),和Tb(III)的特性2,3 / 7,和5 / 9分别。对于两个混合镧系元素复杂系统6和11,根据Eu(III)和Tb(III)的相对含量,可以对发射的颜色进行微调。发现少量的Eu(III)足以观察到Eu(III)最强烈的转变。据信这是同一配合物中从Tb(III)到Eu(III)的能量转移的结果-这一结论得到了Tb(III)寿命显着缩短以及Eu(III)在玻璃中的寿命增加的支持。混合镧系元素络合物相对于具有相同配体的纯Tb(III)和Eu(III)络合物的对应值。


























 京公网安备 11010802027423号
京公网安备 11010802027423号