当前位置:
X-MOL 学术
›
Chem. Heterocycl. Comp.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
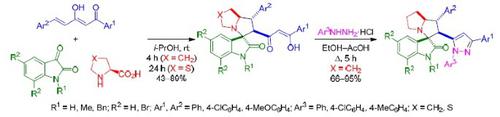
1,5-Diarylpent-4-ene-1,3-diones in the synthesis of spiro[(thia)pyrrolizidine-3,3'-oxindoles] and 1,3-diaryl-5-spiro[oxindole-3,3'-pyrrolizidin-2'-yl]-1 H -pyrazoles
Chemistry of Heterocyclic Compounds ( IF 1.5 ) Pub Date : 2021-02-05 , DOI: 10.1007/s10593-021-02871-0 Vladislav Yu. Korotaev , Nikolay S. Zimnitskiy , Andrey D. Denikaev , Alexey Yu. Barkov , Igor B. Kutyashev , Vyacheslav Ya. Sosnovskikh
中文翻译:

1,5-二芳基戊四烯-1,3-二酮在螺[[thia] pyrolrolizidine-3,3'-oxindoles]和1,3-diaryl-5-spiro [oxindole-3,3'的合成中-吡咯并齐丁-2'-基] -1 H-吡唑类
更新日期:2021-02-05
Chemistry of Heterocyclic Compounds ( IF 1.5 ) Pub Date : 2021-02-05 , DOI: 10.1007/s10593-021-02871-0 Vladislav Yu. Korotaev , Nikolay S. Zimnitskiy , Andrey D. Denikaev , Alexey Yu. Barkov , Igor B. Kutyashev , Vyacheslav Ya. Sosnovskikh
|
The three-component reaction of (E)-1,5-diarylpent-4-ene-1,3-diones, isatins, and (thia)proline in i-PrOH at room temperature proceeds regio- and stereoselectively leading to the formation of spiro[(thia)pyrrolizidine-3,3'-oxindoles] with the 1,3-dicarbonyl moiety in the (thia)pyrrolizidine ring. Subsequent treatment of these tetracyclic products with arylhydrazine hydrochlorides under reflux in the EtOH–AcOH system gave 5-substituted 1,3-diaryl-1H-pyrazoles in high yields.
中文翻译:

1,5-二芳基戊四烯-1,3-二酮在螺[[thia] pyrolrolizidine-3,3'-oxindoles]和1,3-diaryl-5-spiro [oxindole-3,3'的合成中-吡咯并齐丁-2'-基] -1 H-吡唑类
在室温下,i -PrOH中的(E)-1,5-二芳基戊-4-烯-1,3-二酮,靛红和脯氨酸的三组分反应进行区域选择性和立体选择性反应,从而形成螺[(thia)pyrrolizidine-3,3'-oxindoles]在(thia)吡咯烷环上有1,3-二羰基部分。在EtOH-AcOH体系中,在回流下用芳基肼盐酸盐对这些四环产物进行后续处理,可以高产率获得5取代的1,3-二芳基-1 H-吡唑。



























 京公网安备 11010802027423号
京公网安备 11010802027423号