当前位置:
X-MOL 学术
›
Environ. Sci.: Water Res. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Impact of sodium silicate on lead release from lead(II) carbonate
Environmental Science: Water Research & Technology ( IF 5 ) Pub Date : 2021-2-3 , DOI: 10.1039/d0ew00886a Bofu Li 1, 2, 3, 4, 5 , Benjamin F. Trueman 1, 2, 3, 4, 5 , Javier M. Locsin 1, 2, 3, 4, 5 , Yaohuan Gao 1, 2, 3, 4, 5 , Mohammad Shahedur Rahman 1, 2, 3, 4, 5 , Yuri Park 6, 7, 8, 9, 10 , Graham A. Gagnon 1, 2, 3, 4, 5
Environmental Science: Water Research & Technology ( IF 5 ) Pub Date : 2021-2-3 , DOI: 10.1039/d0ew00886a Bofu Li 1, 2, 3, 4, 5 , Benjamin F. Trueman 1, 2, 3, 4, 5 , Javier M. Locsin 1, 2, 3, 4, 5 , Yaohuan Gao 1, 2, 3, 4, 5 , Mohammad Shahedur Rahman 1, 2, 3, 4, 5 , Yuri Park 6, 7, 8, 9, 10 , Graham A. Gagnon 1, 2, 3, 4, 5
Affiliation
|
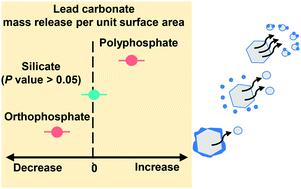
Silicates have been added to drinking water for decades, mainly to control colour by dispersing oxidized iron and manganese. Silicates have been used occasionally to control lead release, but there is no consensus on whether they are effective. Moreover, there are concerns that silicates may disperse particulate lead. We evaluated the effect of sodium silicate on lead release from a model lead(II) carbonate powder using a continuous-flow stirred-tank reactor. We tested a wide range of pH and dissolved inorganic carbon (DIC) concentrations (pH 7.5 or 9, and 5 or 50 mg C per L). We compared sodium silicate against an inhibitor-free control, a better-characterized inhibitor (orthophosphate), and a widely used sequestrant (polyphosphate). Sodium silicate did not have a statistically significant impact on lead release at pH 7.5, regardless of the DIC concentration. At pH 9 it accompanied 80% more lead release at 5 mg C per L and 21% less lead release at 50 mg C per L, compared with controls at matched pH and DIC settings. Sodium silicate did not influence the crystalline phase composition, but it did adsorb to lead(II) carbonate. This may account for its effects at pH 9. Orthophosphate was the more effective inhibitor, yielding 33–96% less lead release than matched controls. Orthophosphate inhibition was attributed to conversion from lead(II) carbonate to hydroxylpyromorphite (Pb5(PO4)3OH) and adsorption to lead(II) carbonate in the absence of a phase conversion (i.e., at pH 9 with 50 mg C per L). Polyphosphate increased lead release by 540–4100%, likely due to aqueous complexation and possibly due to colloidal dispersion.
中文翻译:

硅酸钠对碳酸铅(II)释放铅的影响
硅酸盐已被添加到饮用水中数十年,主要是通过分散氧化铁和锰来控制颜色。硅酸盐偶尔被用来控制铅的释放,但是对于它们是否有效尚无共识。此外,担心硅酸盐可能分散铅颗粒。我们评估了硅酸钠对模型铅中铅释放的影响(II碳酸盐粉末,使用连续流搅拌釜反应器。我们测试了各种pH值和溶解的无机碳(DIC)浓度(pH 7.5或9,每L 5或50 mg C)。我们将硅酸钠与无抑制剂对照,特性更好的抑制剂(正磷酸盐)和广泛使用的螯合剂(聚磷酸盐)进行了比较。不论DIC浓度如何,硅酸钠对pH 7.5下的铅释放没有统计学上的显着影响。在pH 9下,与匹配的pH和DIC设置的对照相比,每升5 mg C的铅释放量增加了80%,每升50 mg C的铅释放量减少了21%。硅酸钠不会影响结晶相组成,但会吸附到铅(II)碳酸盐。这可能是其在pH 9时的影响的原因。正磷酸盐是更有效的抑制剂,铅释放量比匹配的对照少33–96%。正磷酸盐的抑制作用归因于从碳酸铅(II)转化为羟基焦晶石(Pb 5(PO 4)3 OH)和在没有相转化的情况下(即在pH 9下,每毫升含50 mg C )吸附到碳酸铅(II)。L)。聚磷酸盐使铅的释放增加了540-4100%,这可能是由于水的络合和胶体分散所致。
更新日期:2021-02-03
中文翻译:

硅酸钠对碳酸铅(II)释放铅的影响
硅酸盐已被添加到饮用水中数十年,主要是通过分散氧化铁和锰来控制颜色。硅酸盐偶尔被用来控制铅的释放,但是对于它们是否有效尚无共识。此外,担心硅酸盐可能分散铅颗粒。我们评估了硅酸钠对模型铅中铅释放的影响(II碳酸盐粉末,使用连续流搅拌釜反应器。我们测试了各种pH值和溶解的无机碳(DIC)浓度(pH 7.5或9,每L 5或50 mg C)。我们将硅酸钠与无抑制剂对照,特性更好的抑制剂(正磷酸盐)和广泛使用的螯合剂(聚磷酸盐)进行了比较。不论DIC浓度如何,硅酸钠对pH 7.5下的铅释放没有统计学上的显着影响。在pH 9下,与匹配的pH和DIC设置的对照相比,每升5 mg C的铅释放量增加了80%,每升50 mg C的铅释放量减少了21%。硅酸钠不会影响结晶相组成,但会吸附到铅(II)碳酸盐。这可能是其在pH 9时的影响的原因。正磷酸盐是更有效的抑制剂,铅释放量比匹配的对照少33–96%。正磷酸盐的抑制作用归因于从碳酸铅(II)转化为羟基焦晶石(Pb 5(PO 4)3 OH)和在没有相转化的情况下(即在pH 9下,每毫升含50 mg C )吸附到碳酸铅(II)。L)。聚磷酸盐使铅的释放增加了540-4100%,这可能是由于水的络合和胶体分散所致。


























 京公网安备 11010802027423号
京公网安备 11010802027423号