当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
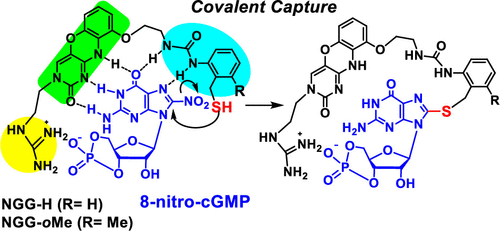
Artificial Host Molecules to Covalently Capture 8-Nitro-cGMP in Neutral Aqueous Solutions and in Cells
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2021-02-02 , DOI: 10.1021/acs.bioconjchem.1c00012 Yasufumi Fuchi 1, 2, 3 , Hirotaka Murase 4 , Ryosuke Kai 1 , Kakeru Kurata 2 , Satoru Karasawa 2 , Shigeki Sasaki 1, 4
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2021-02-02 , DOI: 10.1021/acs.bioconjchem.1c00012 Yasufumi Fuchi 1, 2, 3 , Hirotaka Murase 4 , Ryosuke Kai 1 , Kakeru Kurata 2 , Satoru Karasawa 2 , Shigeki Sasaki 1, 4
Affiliation
|
New 1,3-diazaphenoxazine derivatives (nitroG-Grasp-Guanidine, NGG) have been developed to covalently capture 8-nitro-cGMP in neutral aqueous solutions, which furnish a thiol reactive group to displace the 8-nitro group and a guanidine unit for interaction with the cyclic phosphate. The thiol group was introduced to the 1,3-diazaphenoxazine skeleton through a 2-aminobenzylthiol group (NGG-H) and its 4-methyl (NGG-pMe) and 6-methyl (NGG-oMe) substituted derivatives. The covalent adducts were formed between the NGG derivatives and 8-nitro-cGMP in neutral aqueous solutions. Among the NGG derivatives, the one with the 6-methyl group (NGG-oMe) exhibited the most efficient capture reaction. Furthermore, NGG-H showed a cell permeability into HEK-293 and RAW 264.7 cells and reduced the intracellular 8-nitro-cGMP level. The NGG derivatives developed in this study would become a valuable tool to study the intracellular role of 8-nitro-cGMP.
更新日期:2021-02-17



























 京公网安备 11010802027423号
京公网安备 11010802027423号