当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
CuFeO2–Water Interface under Illumination: Structural, Electronic, and Catalytic Implications for the Hydrogen Evolution Reaction
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-02-03 , DOI: 10.1021/acscatal.0c05066 Matteo Ferri 1 , Joshua David Elliott 2 , Matteo Farnesi Camellone 2 , Stefano Fabris 2 , Simone Piccinin 2
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-02-03 , DOI: 10.1021/acscatal.0c05066 Matteo Ferri 1 , Joshua David Elliott 2 , Matteo Farnesi Camellone 2 , Stefano Fabris 2 , Simone Piccinin 2
Affiliation
|
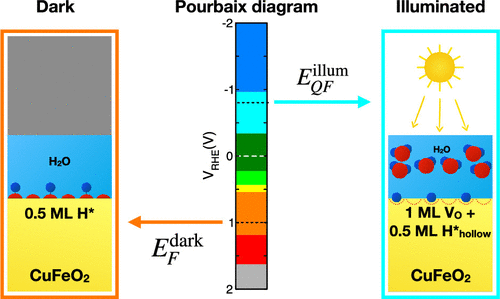
CuFeO2 is a p-type semiconductor that has been recently identified as a promising photocathode material for photoelectrochemical water splitting. CuFeO2 can absorb solar light and promote the hydrogen evolution reaction (HER), even though the photocurrents achieved so far are still well below the theoretical upper limit. While several experimental and theoretical works have provided a detailed characterization of the bulk properties of this material, surfaces have been largely unexplored. In this work, we perform first-principles simulations based on DFT to investigate the structure, electronic properties, and thermodynamic stability of CuFeO2 surfaces both in vacuum and in an electrochemical environment. To estimate the alignment of the band edges on the electrochemical scale, we perform ab initio molecular dynamics in explicit water, unraveling the structure of the solid/liquid interface for various surface terminations. We consider the system both in the dark and under illumination, showing that light absorption can induce partial reduction of the surface, giving rise to states in the gap that can pin the Fermi level, in agreement with recent measurements. Using the free energy of adsorption of atomic hydrogen as a descriptor of the catalytic activity for the HER, we show that hydride species formed at oxygen vacancies can be highly active and could therefore be an intermediate of reaction.
中文翻译:

照明下的CuFeO 2-水界面:氢释放反应的结构,电子和催化意义
CuFeO 2是一种p型半导体,最近已被确定为用于光电化学水分解的有前景的光阴极材料。CuFeO 2可以吸收太阳光并促进氢释放反应(HER),尽管到目前为止获得的光电流仍远低于理论上限。虽然一些实验和理论工作提供了这种材料的整体性能的详细表征,但表面尚未得到广泛的研究。在这项工作中,我们基于DFT进行第一性原理模拟,以研究CuFeO 2的结构,电子性质和热力学稳定性。表面在真空和电化学环境下均可。为了在电化学规模上估计能带边缘的对齐方式,我们在清澈的水中进行了从头算的分子动力学,揭示了各种表面终止反应的固/液界面结构。我们考虑在黑暗和光照下的系统,这表明光吸收可以引起表面的部分还原,从而产生间隙中的状态,从而可以固定费米能级,这与最近的测量结果一致。使用原子氢吸附的自由能作为HER催化活性的指标,我们表明在氧空位处形成的氢化物可能具有很高的活性,因此可能是反应的中间产物。
更新日期:2021-02-19
中文翻译:

照明下的CuFeO 2-水界面:氢释放反应的结构,电子和催化意义
CuFeO 2是一种p型半导体,最近已被确定为用于光电化学水分解的有前景的光阴极材料。CuFeO 2可以吸收太阳光并促进氢释放反应(HER),尽管到目前为止获得的光电流仍远低于理论上限。虽然一些实验和理论工作提供了这种材料的整体性能的详细表征,但表面尚未得到广泛的研究。在这项工作中,我们基于DFT进行第一性原理模拟,以研究CuFeO 2的结构,电子性质和热力学稳定性。表面在真空和电化学环境下均可。为了在电化学规模上估计能带边缘的对齐方式,我们在清澈的水中进行了从头算的分子动力学,揭示了各种表面终止反应的固/液界面结构。我们考虑在黑暗和光照下的系统,这表明光吸收可以引起表面的部分还原,从而产生间隙中的状态,从而可以固定费米能级,这与最近的测量结果一致。使用原子氢吸附的自由能作为HER催化活性的指标,我们表明在氧空位处形成的氢化物可能具有很高的活性,因此可能是反应的中间产物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号