当前位置:
X-MOL 学术
›
Clean - Soil Air Water
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Arsenic Adsorption on Modified Clay Minerals in Contaminated Soil and Water: Impact of pH and Competitive Anions
Clean - Soil Air Water ( IF 1.7 ) Pub Date : 2021-01-27 , DOI: 10.1002/clen.202000259 Raj Mukhopadhyay 1, 2 , Binoy Sarkar 3 , Arijit Barman 1, 2 , Samar Chandra Datta 2 , Kanchikeri Math Manjaiah 2
Clean - Soil Air Water ( IF 1.7 ) Pub Date : 2021-01-27 , DOI: 10.1002/clen.202000259 Raj Mukhopadhyay 1, 2 , Binoy Sarkar 3 , Arijit Barman 1, 2 , Samar Chandra Datta 2 , Kanchikeri Math Manjaiah 2
Affiliation
|
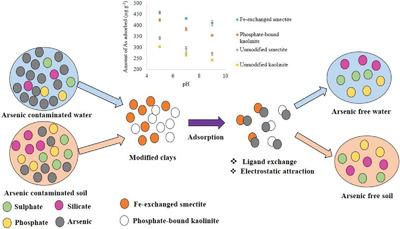
This study evaluates the arsenic adsorption behavior of Fe‐exchanged smectite and phosphate‐bound kaolinite, in soil, tap water and double distilled water in the presence of competing anions such as silicate, phosphate, and sulfate, and at variable pH values. The maximum amounts of As adsorbed in soil are 620.6 and 607.6 µg g–1 at pH 5 by Fe‐exchanged smectite and phosphate‐bound kaolinite, respectively. The pH‐modified Freundlich equation fits well (R2 > 0.96) to the adsorption data, distinguishing the effect of pH on adsorption. The coefficients of pH‐value are 0.04 and 0.05 for phosphate‐bound kaolinite and Fe‐exchanged smectite, suggesting that low pH is suitable for the adsorption. The As adsorption is decreased in tap water at low pH compared to the soil due to the presence of iron (Fe2+/3+), sulfate, and bicarbonate in tap water. Among the competing anions in distilled water, phosphate is the most interfering anion for As adsorption. The competition coefficients of As‐phosphate binary adsorption derived from the Sheindorf equation are 3.93 and 0.56 for Fe‐exchanged smectite and phosphate‐bound kaolinite at pH 5. The Fe‐exchanged smectite can be used more effectively than phosphate‐bound kaolinite for As remediation in systems having low pH (pH ≈5) and high phosphate concentration.
中文翻译:

砷在被污染的土壤和水中的改性粘土矿物上的吸附:pH和竞争性阴离子的影响
这项研究评估了在存在竞争性阴离子(例如硅酸盐,磷酸盐和硫酸盐)且pH值可变的情况下,铁交换蒙脱石和磷酸盐结合的高岭石在土壤,自来水和双重蒸馏水中的砷吸附行为。Fe交换蒙脱石和磷酸盐结合的高岭石在pH值为5时在土壤中吸附的As的最大量分别为620.6和607.6 µg g –1。pH修正的Freundlich方程拟合得很好(R 2> 0.96)的吸附数据,以区分pH对吸附的影响。磷酸盐结合的高岭石和铁交换的蒙脱石的pH值系数分别为0.04和0.05,这表明低pH值适合于吸附。与土壤相比,由于土壤中铁(Fe 2 + / 3 +),自来水中的硫酸盐和碳酸氢盐。在蒸馏水中竞争的阴离子中,磷酸根是对As吸附的最大干扰阴离子。由Sheindorf方程得出的As-磷酸盐二元吸附在pH 5时对Fe交换的蒙脱石和磷酸盐结合的高岭石的竞争系数分别为3.93和0.56。Fe交换的蒙脱石比磷酸盐结合的高岭石可更有效地用于As的修复。在低pH(pH≈5)和高磷酸盐浓度的系统中。
更新日期:2021-01-27
中文翻译:

砷在被污染的土壤和水中的改性粘土矿物上的吸附:pH和竞争性阴离子的影响
这项研究评估了在存在竞争性阴离子(例如硅酸盐,磷酸盐和硫酸盐)且pH值可变的情况下,铁交换蒙脱石和磷酸盐结合的高岭石在土壤,自来水和双重蒸馏水中的砷吸附行为。Fe交换蒙脱石和磷酸盐结合的高岭石在pH值为5时在土壤中吸附的As的最大量分别为620.6和607.6 µg g –1。pH修正的Freundlich方程拟合得很好(R 2> 0.96)的吸附数据,以区分pH对吸附的影响。磷酸盐结合的高岭石和铁交换的蒙脱石的pH值系数分别为0.04和0.05,这表明低pH值适合于吸附。与土壤相比,由于土壤中铁(Fe 2 + / 3 +),自来水中的硫酸盐和碳酸氢盐。在蒸馏水中竞争的阴离子中,磷酸根是对As吸附的最大干扰阴离子。由Sheindorf方程得出的As-磷酸盐二元吸附在pH 5时对Fe交换的蒙脱石和磷酸盐结合的高岭石的竞争系数分别为3.93和0.56。Fe交换的蒙脱石比磷酸盐结合的高岭石可更有效地用于As的修复。在低pH(pH≈5)和高磷酸盐浓度的系统中。



























 京公网安备 11010802027423号
京公网安备 11010802027423号