当前位置:
X-MOL 学术
›
Helv. Chimica Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Probing BRD Inhibition Substituent Effects in Bulky Analogues of (+)‐JQ1
Helvetica Chimica Acta ( IF 1.8 ) Pub Date : 2021-01-27 , DOI: 10.1002/hlca.202000214 John Spencer 1 , Storm Hassell 2 , Sarah Picaud 3 , Ralph Lengacher 4 , Joshua Csuker 4 , Regis Millet 5 , Gilles Gasser 6 , Roger Alberto 7 , Hannah Maple 8 , Robert Felix 8 , Zbigniew Leśnikowski 9 , Helen Stewart 10 , Timothy Chevassut 11 , Simon Morley 12 , Panagis Filippakopoulos 3
Helvetica Chimica Acta ( IF 1.8 ) Pub Date : 2021-01-27 , DOI: 10.1002/hlca.202000214 John Spencer 1 , Storm Hassell 2 , Sarah Picaud 3 , Ralph Lengacher 4 , Joshua Csuker 4 , Regis Millet 5 , Gilles Gasser 6 , Roger Alberto 7 , Hannah Maple 8 , Robert Felix 8 , Zbigniew Leśnikowski 9 , Helen Stewart 10 , Timothy Chevassut 11 , Simon Morley 12 , Panagis Filippakopoulos 3
Affiliation
|
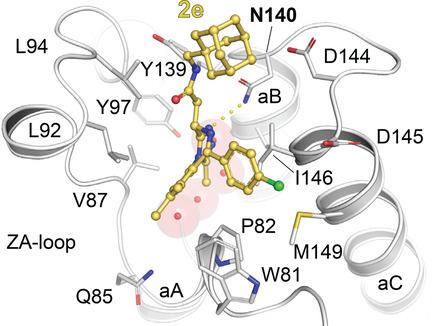
A series of bulky organometallic and organic analogues of the bromodomain (BRD) inhibitor (+)‐JQ1 have been prepared. The most potent, N‐[(adamantan‐1‐yl)methyl]‐2‐[(9S)‐7‐(4‐chlorophenyl)‐4,5,13‐trimethyl‐3‐thia‐1,8,11,12‐tetraazatricyclo[8.3.0.02,6]trideca‐2(6),4,7,10,12‐pentaen‐9‐yl]acetamide, 2e, showed excellent potency with an KD=ca. 130 nm vs. BRD4(1) and a ca. 2‐fold selectivity over BRD4(2) (KD=ca. 260 nm). Its binding to the first bromodomain of BRD4 was determined by a protein cocrystal structure.
中文翻译:

在(+)‐ JQ1的大型类似物中探索BRD抑制取代基的作用
已经准备了一系列大的溴结构域(BRD)抑制剂(+)-JQ1的有机金属和有机类似物。最有效的N -[(金刚烷-1-基)甲基] -2-[(9 S)-7-(4-氯苯基)-4,5,13-三甲基-3-硫杂1,8,11 ,12-四氮杂[8.3.0.02,6]十三-2(6),4,7,10,12五烯-9-基]乙酰胺,2E,表现出优异的效力与ķ d = CA。为130N米 VS。BRD4(1)和CA。选择性是BRD4(2)的2倍(K D =约260 n m)。它与BRD4的第一个溴结构域的结合是通过蛋白质共晶体结构确定的。
更新日期:2021-03-10
中文翻译:

在(+)‐ JQ1的大型类似物中探索BRD抑制取代基的作用
已经准备了一系列大的溴结构域(BRD)抑制剂(+)-JQ1的有机金属和有机类似物。最有效的N -[(金刚烷-1-基)甲基] -2-[(9 S)-7-(4-氯苯基)-4,5,13-三甲基-3-硫杂1,8,11 ,12-四氮杂[8.3.0.02,6]十三-2(6),4,7,10,12五烯-9-基]乙酰胺,2E,表现出优异的效力与ķ d = CA。为130N米 VS。BRD4(1)和CA。选择性是BRD4(2)的2倍(K D =约260 n m)。它与BRD4的第一个溴结构域的结合是通过蛋白质共晶体结构确定的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号