当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Advances in Transition‐Metal Catalyzed Carbonylative Suzuki‐Miyaura Coupling Reaction: An Update
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-01-25 , DOI: 10.1002/adsc.202001509 Dhananjay Bhattacherjee 1 , Matiur Rahman 1 , Sumit Ghosh 2 , Avik Kumar Bagdi 3 , Grigory V. Zyryanov 1, 4 , Oleg N. Chupakhin 1, 4 , Pralay Das 5, 6 , Alakananda Hajra 2
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-01-25 , DOI: 10.1002/adsc.202001509 Dhananjay Bhattacherjee 1 , Matiur Rahman 1 , Sumit Ghosh 2 , Avik Kumar Bagdi 3 , Grigory V. Zyryanov 1, 4 , Oleg N. Chupakhin 1, 4 , Pralay Das 5, 6 , Alakananda Hajra 2
Affiliation
|
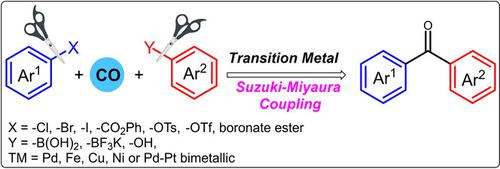
Transition metals have been an indispensable component of modern catalysis and among these palladium is the top‐ranked choice of chemists as a catalyst. After the several important developments of palladium catalysed C−C cross‐coupling reactions, carbonylative transformations have been realised as an attractive post modification of these reactions. Carbonylative Suzuki‐Miyaura coupling reaction is among such transformations for direct incorporation of CO fragment for the preparation of biaryl or aryl/alkyl ketones via transition‐metal catalysis. Ketone functionality is basically a valuable building block having huge pharmaceutical and agrochemical applications. Moreover, carbonylative Suzuki‐Miyaura coupling is also used in the synthesis of various natural bioactive molecules through the direct joining of molecular fragments via CO bridge. In this review, the past decade developments in the carbonylative Suzuki‐Miyaura coupling reactions are documented in detail with modern approaches in transition‐metal catalysis.
中文翻译:

过渡金属催化的羰基化铃木-宫浦偶联反应的研究进展
过渡金属一直是现代催化中必不可少的组成部分,其中钯是化学家最优先选择的催化剂。在钯催化的CC交叉偶联反应的一些重要发展之后,羰基化转化已经成为这些反应的一种有吸引力的后修饰。Carbonylative铃木-宫浦偶合反应是这样的转化为用于制备联芳基或芳基/烷基酮的CO片段的直接掺入之间经由过渡金属催化。酮功能基本上是具有巨大的制药和农业化学应用的有价值的构建基块。此外,羰基化的Suzuki-Miyaura偶联还可以通过CO桥直接连接分子片段,从而用于合成各种天然生物活性分子。在这篇综述中,用过渡金属催化的现代方法详细记录了羰基化Suzuki-Miyaura偶联反应在过去十年中的发展。
更新日期:2021-03-16
中文翻译:

过渡金属催化的羰基化铃木-宫浦偶联反应的研究进展
过渡金属一直是现代催化中必不可少的组成部分,其中钯是化学家最优先选择的催化剂。在钯催化的CC交叉偶联反应的一些重要发展之后,羰基化转化已经成为这些反应的一种有吸引力的后修饰。Carbonylative铃木-宫浦偶合反应是这样的转化为用于制备联芳基或芳基/烷基酮的CO片段的直接掺入之间经由过渡金属催化。酮功能基本上是具有巨大的制药和农业化学应用的有价值的构建基块。此外,羰基化的Suzuki-Miyaura偶联还可以通过CO桥直接连接分子片段,从而用于合成各种天然生物活性分子。在这篇综述中,用过渡金属催化的现代方法详细记录了羰基化Suzuki-Miyaura偶联反应在过去十年中的发展。

























 京公网安备 11010802027423号
京公网安备 11010802027423号