Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cospatial σ-Hole and Lone Pair Interactions of Square-Pyramidal Pentavalent Halogen Compounds with π-Systems: A Quantum Mechanical Study
ACS Omega ( IF 4.1 ) Pub Date : 2021-01-24 , DOI: 10.1021/acsomega.0c05795 Mahmoud A. A. Ibrahim 1 , Ossama A. M. Ahmed 1, 2 , Sabry El-Taher 2 , Jabir H. Al-Fahemi 3 , Nayra A. M. Moussa 1 , Hussein Moustafa 2
ACS Omega ( IF 4.1 ) Pub Date : 2021-01-24 , DOI: 10.1021/acsomega.0c05795 Mahmoud A. A. Ibrahim 1 , Ossama A. M. Ahmed 1, 2 , Sabry El-Taher 2 , Jabir H. Al-Fahemi 3 , Nayra A. M. Moussa 1 , Hussein Moustafa 2
Affiliation
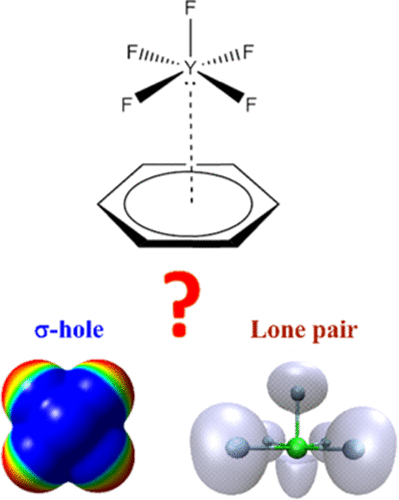
|
In the spirit of the mounting interest in noncovalent interactions, the present study was conducted to scrutinize a special type that simultaneously involved both σ-hole and lone pair (lp) interactions with aromatic π-systems. Square-pyramidal pentavalent halogen-containing molecules, including X-Cl-F4, F-Y-F4, and F-I-X4 compounds (where X = F, Cl, Br, and I and Y = Cl, Br, and I) were employed as σ-hole/lp donors. On the other hand, benzene (BZN) and hexafluorobenzene (HFB) were chosen as electron-rich and electron-deficient aromatic π-systems, respectively. The investigation relied upon a variety of quantum chemical calculations that complement each other. The results showed that (i) the binding energy of the X-Y-F4···BZN complexes increased (i.e., more negative) as the Y atom had a larger magnitude of σ-hole, contrary to the pattern of X-Y-F4···HFB complexes; (ii) the interaction energies of X-Y-F4···BZN complexes were dominated by both dispersion and electrostatic contributions, while dispersive interactions dominated X-Y-F4···HFB complexes; and (iii) the X4 atoms in F-I-X4···π-system complexes governed the interaction energy pattern: the larger the X4 atoms were, the greater the interaction energies were, for the same π-system. The results had illuminating facets in regard to the rarely addressed cases of the σ-hole/lp contradictory scene.
中文翻译:

方形金字塔形五价卤素化合物与π系统的共空间σ孔和孤对相互作用:量子力学研究
本着对非共价相互作用越来越感兴趣的精神,进行了本研究以研究一种特殊类型,该类型同时涉及与芳香族π系统的σ孔和孤对(lp)相互作用。使用包括X-Cl-F 4,FYF 4和FIX 4化合物(其中X = F,Cl,Br和I且Y = Cl,Br和I)的正方形金字塔形五价含卤素分子作为σ -孔/ lp供体。另一方面,分别选择苯(BZN)和六氟苯(HFB)作为富电子和缺电子的芳族π系统。研究依赖于相互补充的各种量子化学计算。结果表明:(i)XYF 4的结合能当Y原子具有更大的σ孔大小时,BZN络合物增加(即,更负),这与XYF 4· HFB络合物的模式相反;(ii)XYF 4 ···BZN配合物的相互作用能主要由色散和静电作用决定,而色散相互作用则主要由XY-F4···HFB配合物决定;和(iii)在X 4个中FIX原子4 ···π系统络合物管辖的相互作用能量图案:越大X 4个原子是,相互作用能是较大的,对于相同的π体系。关于σ-hole/ lp矛盾场景很少解决的情况,结果具有启发性的方面。
更新日期:2021-02-02
中文翻译:

方形金字塔形五价卤素化合物与π系统的共空间σ孔和孤对相互作用:量子力学研究
本着对非共价相互作用越来越感兴趣的精神,进行了本研究以研究一种特殊类型,该类型同时涉及与芳香族π系统的σ孔和孤对(lp)相互作用。使用包括X-Cl-F 4,FYF 4和FIX 4化合物(其中X = F,Cl,Br和I且Y = Cl,Br和I)的正方形金字塔形五价含卤素分子作为σ -孔/ lp供体。另一方面,分别选择苯(BZN)和六氟苯(HFB)作为富电子和缺电子的芳族π系统。研究依赖于相互补充的各种量子化学计算。结果表明:(i)XYF 4的结合能当Y原子具有更大的σ孔大小时,BZN络合物增加(即,更负),这与XYF 4· HFB络合物的模式相反;(ii)XYF 4 ···BZN配合物的相互作用能主要由色散和静电作用决定,而色散相互作用则主要由XY-F4···HFB配合物决定;和(iii)在X 4个中FIX原子4 ···π系统络合物管辖的相互作用能量图案:越大X 4个原子是,相互作用能是较大的,对于相同的π体系。关于σ-hole/ lp矛盾场景很少解决的情况,结果具有启发性的方面。



























 京公网安备 11010802027423号
京公网安备 11010802027423号