当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Formal Semisynthesis of Demethylgorgosterol Utilizing a Stereoselective Intermolecular Cyclopropanation Reaction
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2021-01-23 , DOI: 10.1002/ejoc.202100035 Nicolai Rosenbaum 1 , Lisa Schmidt 2 , Florian Mohr 3 , Olaf Fuhr 3 , Martin Nieger 4 , Stefan Bräse 5
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2021-01-23 , DOI: 10.1002/ejoc.202100035 Nicolai Rosenbaum 1 , Lisa Schmidt 2 , Florian Mohr 3 , Olaf Fuhr 3 , Martin Nieger 4 , Stefan Bräse 5
Affiliation
|
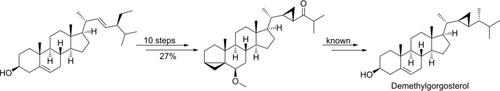
A formal semisynthesis of the marine steroid demethylgorgosterol was realized by the synthesis of an advanced ketone intermediate. The intermediate was synthesized in ten steps, starting from commercially available stigmasterol. An overall yield of 27 % was achieved. The key step was a stereoselective cyclopropanation reaction with 82 % yield, a trans/cis ratio of 89 : 11, and drtrans of >99 : 1.

中文翻译:

利用立体选择性分子间环丙烷化反应形式的脱甲基庚固醇的形式半合成
通过合成高级酮中间体,可以实现海洋类固醇脱甲基庚固醇的正式半合成。从市售的豆甾醇开始十步合成中间体。总产率达到27%。关键步骤是立体选择性环丙烷化反应,收率为82%,反式/顺式比为89:11,dr反式> 99:1。

更新日期:2021-03-08

中文翻译:

利用立体选择性分子间环丙烷化反应形式的脱甲基庚固醇的形式半合成
通过合成高级酮中间体,可以实现海洋类固醇脱甲基庚固醇的正式半合成。从市售的豆甾醇开始十步合成中间体。总产率达到27%。关键步骤是立体选择性环丙烷化反应,收率为82%,反式/顺式比为89:11,dr反式> 99:1。




























 京公网安备 11010802027423号
京公网安备 11010802027423号