当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic Understanding of Base‐Catalyzed Aldimine/Ketoamine Condensations: An Old Story and A New Model
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2021-01-23 , DOI: 10.1002/ajoc.202000700 Zhe‐wei Li 1 , Lin Zhang 1 , Min Pu 1 , Ming Lei 1
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2021-01-23 , DOI: 10.1002/ajoc.202000700 Zhe‐wei Li 1 , Lin Zhang 1 , Min Pu 1 , Ming Lei 1
Affiliation
|
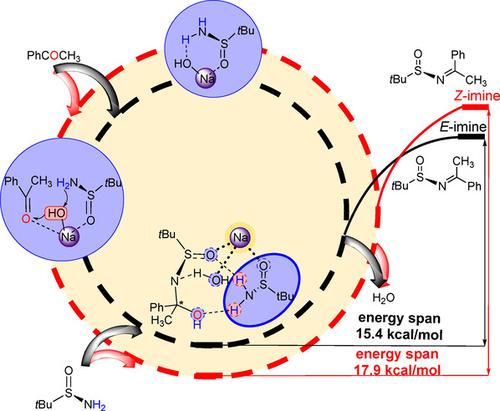
Aldimine/ketoamine condensations are basic reactions in chemical synthesis. Herein a density functional theory (DFT) study was performed to characterize the mechanism of the ketoamine condensation of acetophenone with tert‐butanesulfinamide catalyzed by NaOH. The calculated results showed that the main N‐tert‐butanesulfinimines products own E configuration, which are due to both thermodynamic and kinetic effects. Meanwhile, the key roles of the substrate cage and the synergistic catalytic mode formed by metal cation and hydroxyl anion were pointed out, which imply the influence of noncovalent interactions on reaction activity. This theoretical study revealed the origin of E sulfinimines achieved in aldimine/ketoamine condensations catalyzed by base, which could provide new insights into the nature for the condensation reactions.
中文翻译:

对碱催化的醛亚胺/酮胺缩合的机械理解:一个古老的故事和一个新的模型
醛亚胺/酮胺缩合是化学合成中的基本反应。本文进行了密度泛函理论(DFT)研究,以表征苯乙酮与NaOH催化的叔丁烷亚磺酰胺的酮胺缩合机理。计算结果表明,主要的N-叔丁烷亚磺胺产品具有E构型,这是由于热力学和动力学效应所致。同时指出了底物笼的关键作用以及金属阳离子与羟基阴离子形成的协同催化模式,暗示了非共价相互作用对反应活性的影响。这项理论研究揭示了E的起源 在碱催化的醛亚胺/酮胺缩合反应中获得的亚砜亚胺可为缩合反应的性质提供新的见解。
更新日期:2021-03-11
中文翻译:

对碱催化的醛亚胺/酮胺缩合的机械理解:一个古老的故事和一个新的模型
醛亚胺/酮胺缩合是化学合成中的基本反应。本文进行了密度泛函理论(DFT)研究,以表征苯乙酮与NaOH催化的叔丁烷亚磺酰胺的酮胺缩合机理。计算结果表明,主要的N-叔丁烷亚磺胺产品具有E构型,这是由于热力学和动力学效应所致。同时指出了底物笼的关键作用以及金属阳离子与羟基阴离子形成的协同催化模式,暗示了非共价相互作用对反应活性的影响。这项理论研究揭示了E的起源 在碱催化的醛亚胺/酮胺缩合反应中获得的亚砜亚胺可为缩合反应的性质提供新的见解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号