Tetrahedron Letters ( IF 1.8 ) Pub Date : 2021-01-23 , DOI: 10.1016/j.tetlet.2021.152827 Nannaphat Chumsri , Chutima Kuhakarn , Pawaret Leowanawat , Vichai Reutrakul , Darunee Soorukram
|
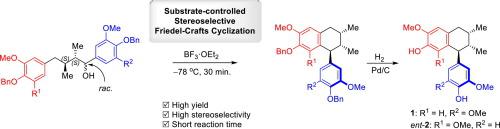
A concise asymmetric synthesis of naturally occurring bioactive 2,7′-cyclolignans, namely 4,4′-dihydroxy-3′,5,5′-trimethoxy-2,7′-cyclolignan and 4,4′-dihydroxy-3,3′,5-trimethoxy-2,7′-cyclolignan, possessing the uncommon 8,8′-syn dimethyl stereochemistry and unsymmetrical C-6 units with 7′,8′-anti-orientation is described using a substrate-controlled stereoselective Friedel-Crafts cyclization as the key step. The products were obtained in good yields with high stereoselectivity. The absolute configurations of natural 4,4′-dihydroxy-3′,5,5′-trimethoxy-2,7′-cyclolignan and those of natural 4,4′-dihydroxy-3,3′,5-trimethoxy-2,7′-cyclolignan were assigned as (7′S,8S,8′S) and (7′R,8R,8′R), respectively, based on the experimental circular dichroism (CD) spectra of the corresponding synthesized compounds.
中文翻译:

简明合成和天然存在的生物活性2,7'-cyclolignans的绝对构型的确认
天然存在的生物活性2,7'-cyclolignans的简明不对称合成,即4,4'-dihydroxy-3',5,5'-trimethoxy-2,7'-cyclolignan和4,4'-dihydroxy-3,3使用受底物控制的立体选择性Friedel- F描述了具有罕见的8,8'-顺二甲基立体化学和具有7',8'-反取向的不对称C-6单元的',5-三甲氧基-2,7'-cyclolignan。工艺环化是关键步骤。以高产率获得具有高立体选择性的产物。天然4,4'-二羟基-3',5,5'-三甲氧基-2,7'-cyclolignan和天然4,4'-二羟基-3,3',5-三甲氧基-2的绝对构型7'-cyclolignan被指定为(7 'S,8 S,8 'S)和(7'R,8R,8'R),分别基于相应合成化合物的实验圆二色性(CD)光谱。



























 京公网安备 11010802027423号
京公网安备 11010802027423号