Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2021-01-23 , DOI: 10.1016/j.bmc.2021.116034 Masahiro Kamaura 1 , Osamu Kubo 1 , Hiromichi Sugimoto 1 , Naoyoshi Noguchi 1 , Hirohisa Miyashita 1 , Shinichi Abe 1 , Kae Matsuda 1 , Yoshiyuki Tsujihata 1 , Tomoyuki Odani 2 , Shinji Iwasaki 3 , Toshiki Murata 1 , Kenjiro Sato 1
|
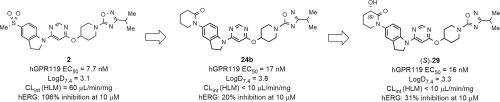
We previously identified a novel series of indolinylpyrimidine derivatives exemplified by 2 in Figure 1, which is an indoline based derivative, as potent GPR119 agonists. Despite the attractive potency of 2, this compound inhibited the human ether-a-go-go-related gene (hERG) K+ channel. We elucidated crucial roles of the methylsulfonyl group of 2 in its interaction with the hERG channel and the GPR119 receptor, presumably as a hydrogen bond acceptor (HBA). To remove the undesirable hERG inhibitory activity, a strategy was implemented to arrange an HBA on a less conformationally flexible framework at the indoline 5-position instead of the methylsulfonyl group. This successfully led to the discovery of a piperidinone ring as a desirable motif at the indoline 5-position, which could minimize hERG liability as shown by 24b. Further optimization focused on the reduction of lipophilicity in terms of more favorable drug-like properties. Consequently, the introduction of a hydroxy group at the 3-position of the piperidinone ring effectively reduced lipophilicity without compromising GPR119 potency, resulting in the identification of (3S)-3-hydroxy-1-{1-[6-({1-[3-(propan-2-yl)-1,2,4-oxadiazol-5-yl]piperidin-4-yl}oxy)pyrimidin-4-yl]- 2,3-dihydro-1H-indol-5-yl}piperidin-2-one ((S)-29) as a novel, potent, and orally bioavailable GPR119 agonist with a well-balanced profile. The pharmacological effects of this compound were also confirmed after single and chronic oral administration in diabetic animal models.
中文翻译:

发现一系列基于吲哚基嘧啶的新型 GPR119 激动剂:使用以氢键受体为中心的方法消除与 ether-a-go-go 相关的基因责任
我们之前确定了一系列新的二氢吲哚嘧啶衍生物,如图 1 中的 2 所示,它是一种基于二氢吲哚的衍生物,可作为有效的 GPR119 激动剂。尽管 2 具有吸引力,但该化合物抑制了人类 ether-a-go-go 相关基因 (hERG) K +渠道。我们阐明了 2 的甲基磺酰基在其与 hERG 通道和 GPR119 受体相互作用中的关键作用,大概是作为氢键受体 (HBA)。为了消除不需要的 hERG 抑制活性,实施了一种策略,将 HBA 安排在二氢吲哚 5 位的构象灵活性较差的框架上,而不是甲基磺酰基。这成功地导致发现哌啶酮环作为二氢吲哚 5 位的理想基序,这可以最大限度地减少 hERG 责任,如 24b 所示。进一步优化的重点是降低亲脂性,以获得更有利的类药物特性。因此,在哌啶酮环的 3 位引入羟基有效地降低了亲脂性,而不会影响 GPR119 的效力,S )-3-羟基-1-{1-[6-({1-[3-(丙-2-基)-1,2,4-恶二唑-5-基]哌啶-4-基}氧基) pyrimidin-4-yl]- 2,3-dihydro-1 H -indol-5-yl}piperidin-2-one (( S )-29) 作为一种新型的、有效的、口服生物可利用的 GPR119 激动剂,具有良好的平衡轮廓。在糖尿病动物模型中单次和长期口服给药后,也证实了该化合物的药理作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号