当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Evaluation of the Pr + O → PrO+ + e− chemi-ionization reaction enthalpy and praseodymium oxide, carbide, dioxide, and carbonyl cation bond energies
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2021-1-18 , DOI: 10.1039/d0cp06252a Maryam Ghiassee 1, 2, 3, 4 , Brandon C. Stevenson 1, 2, 3, 4 , P. B. Armentrout 1, 2, 3, 4
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2021-1-18 , DOI: 10.1039/d0cp06252a Maryam Ghiassee 1, 2, 3, 4 , Brandon C. Stevenson 1, 2, 3, 4 , P. B. Armentrout 1, 2, 3, 4
Affiliation
|
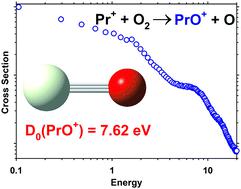
Guided ion beam tandem mass spectrometry (GIBMS) was used to measure the kinetic energy dependent product ion cross sections for reactions of the lanthanide metal praseodymium cation (Pr+) with O2, CO2, and CO and reactions of PrO+ with CO, O2, and Xe. PrO+ is formed through barrierless exothermic processes when the atomic metal cation reacts with O2 and CO2, whereas all other reactions are observed to be endothermic. Analyses of the kinetic energy dependences of these cross sections yield 0 K bond dissociation energies (BDEs) for PrO+, PrC+, PrCO+, and PrO2+. The 0 K BDE for PrO+ is determined to be 7.62 ± 0.09 eV from the weighted average of five independent thresholds. This value is combined with the well-established ionization energy (IE) of Pr to indicate an exothermicity of the chemi-ionization reaction, Pr + O → PrO+ + e−, of 2.15 ± 0.09 eV. Additionally, BDEs of Pr+–C, OPr+–O, and Pr+–CO are determined to be 2.97 ± 0.10. 2.47 ± 0.11, and 0.31 ± 0.07 eV. Theoretical Pr+–O, Pr+–C, OPr+–O, and Pr+–CO BDEs are calculated for comparison with experimental values. The Pr+–O BDE is underestimated at the B3LYP and PBE0 level of theory but better agreement is obtained using the coupled-cluster with single, double, and perturbative triple excitations, CCSD(T), level. Density functional theory approaches yield better agreement for the BDEs of Pr+–C, OPr+–O, and Pr+–CO.
中文翻译:

Pr + O→PrO + + e-化学电离反应焓和oxide氧化物,碳化物,二氧化物和羰基阳离子键能的评估
引导离子束串联质谱(GIBMS)用于测量镧系元素金属ody阳离子(Pr +)与O 2,CO 2和CO的反应以及PrO +与CO的反应的动能依赖性产物离子截面, O 2和Xe。当原子金属阳离子与O 2和CO 2反应时,PrO +是通过无障碍放热过程形成的,而所有其他反应都被认为是吸热的。这些横截面的动能依赖性的分析收率为Pro 0 K时键离解能(二苯醚)+,PRC +,濮耐+和PrO 2 +。从五个独立阈值的加权平均值确定PrO +的0 K BDE为7.62±0.09 eV。这个值与成熟的电离能量Pr的(IE)结合,以指示化学-电离反应的放热性,镨+ O→了PrO + + E - 2.15±0.09电子伏特。另外,确定Pr + –C,OPr + –O和Pr + –CO的BDE为2.97±0.10。2.47±0.11和0.31±0.07 eV。计算理论上的Pr + –O,Pr + –C,OPr + –O和Pr + –CO BDE与实验值进行比较。Pr +-O BDE在理论上的B3LYP和PBE0水平上被低估,但使用具有单,双和扰动三重激发CCSD(T)水平的耦合簇,可以获得更好的一致性。密度泛函理论方法对于Pr + –C,OPr + –O和Pr + –CO的BDE具有更好的一致性。
更新日期:2021-01-22
中文翻译:

Pr + O→PrO + + e-化学电离反应焓和oxide氧化物,碳化物,二氧化物和羰基阳离子键能的评估
引导离子束串联质谱(GIBMS)用于测量镧系元素金属ody阳离子(Pr +)与O 2,CO 2和CO的反应以及PrO +与CO的反应的动能依赖性产物离子截面, O 2和Xe。当原子金属阳离子与O 2和CO 2反应时,PrO +是通过无障碍放热过程形成的,而所有其他反应都被认为是吸热的。这些横截面的动能依赖性的分析收率为Pro 0 K时键离解能(二苯醚)+,PRC +,濮耐+和PrO 2 +。从五个独立阈值的加权平均值确定PrO +的0 K BDE为7.62±0.09 eV。这个值与成熟的电离能量Pr的(IE)结合,以指示化学-电离反应的放热性,镨+ O→了PrO + + E - 2.15±0.09电子伏特。另外,确定Pr + –C,OPr + –O和Pr + –CO的BDE为2.97±0.10。2.47±0.11和0.31±0.07 eV。计算理论上的Pr + –O,Pr + –C,OPr + –O和Pr + –CO BDE与实验值进行比较。Pr +-O BDE在理论上的B3LYP和PBE0水平上被低估,但使用具有单,双和扰动三重激发CCSD(T)水平的耦合簇,可以获得更好的一致性。密度泛函理论方法对于Pr + –C,OPr + –O和Pr + –CO的BDE具有更好的一致性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号