当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemical characterization and thermodynamic analysis of TEMPO derivatives in ionic liquids
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2021-1-7 , DOI: 10.1039/d0cp05350c Luke Wylie 1, 2, 3, 4 , Kan Hakatayama-Sato 5, 6, 7, 8 , Choitsu Go 5, 6, 7, 8 , Kenichi Oyaizu 5, 6, 7, 8 , Ekaterina I. Izgorodina 1, 2, 3, 4
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2021-1-7 , DOI: 10.1039/d0cp05350c Luke Wylie 1, 2, 3, 4 , Kan Hakatayama-Sato 5, 6, 7, 8 , Choitsu Go 5, 6, 7, 8 , Kenichi Oyaizu 5, 6, 7, 8 , Ekaterina I. Izgorodina 1, 2, 3, 4
Affiliation
|
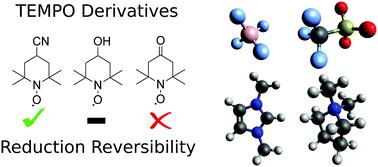
In this study we investigate the reversibility of the reduction process of three TEMPO derivatives – TEMPOL, 4-cyano-TEMPO, and 4-oxo-TEMPO. The [C2mim][BF4] and [C4mpyr][OTf] ionic liquids (ILs) were used to perform cyclic voltammetry (CV) to analyse the redox potentials of the TEMPO derivatives. The former was previously shown to quench the aminoxy anion of TEMPO through a proton transfer reaction with the cation, whereas the latter supported the irreversibility of the TEMPO reduction process. In CV results on TEMPO derivatives, it was shown that [C4mpyr][OTf] could allow for a high degree of reversibility in the reduction of 4-cyano-TEMPO and a moderate degree of reversibility in the reduction of TEMPOL. In comparison, reduction of 4-cyano-TEMPO was predominantly irreversible in [C2mim][BF4], whilst TEMPOL showed complete irreversibility. 4-Oxo-TEMPO did not show any notable reduction reversibility in either IL tested. Reduction potentials showed little variation between the derivatives and 0.2 V variation between the ILs, with the most negative reduction potential being observed at −1.43 V vs. Fc/Fc+ for TEMPOL in [C4mpyr][OTf]. To explain the varying degrees of reversibility of the reduction process, four types of side reactions involving proton transfer to the aminoxy anion were studied using highly correlated quantum chemical methods. Proton transfer from the IL cation was shown to have the ability to quench all three aminoxy anions depending on the IL used. On average, TEMPOL was shown to be the most susceptible to proton transfer from the IL cation, having an average Gibbs free energy (GFE) of 10.5 kJ mol−1 more negative than that of 4-cyano-TEMPO, which was shown to have the highest GFE of proton transfer. Side reactions between water and aminoxy anions were also seen to have the potential to contribute to degradation of the aminoxy anions tested, with 4-oxo-TEMPO being shown to be the most reactive to degradation with water with a GFE of −12.6 kJ mol−1. 4-Oxo-TEMPO was found to be highly susceptible to self-quenching by its aminoxy anion and radical form with highly negative proton transfer GFEs of −47.9 kJ mol−1 and −57.7 kJ mol−1, respectively. Overall, 4-cyano-TEMPO is recommended as being the most stable of the aminoxy anions tested with TEMPOL, thus providing a viable alternative to improve solubility should the IL be tuned to maximize its stability.
中文翻译:

TEMPO衍生物在离子液体中的电化学表征和热力学分析
在这项研究中,我们研究了三种TEMPO衍生物-TEMPOL,4-氰基-TEMPO和4-氧代-TEMPO的还原过程的可逆性。使用[C 2 mim] [BF 4 ]和[C 4 mpyr] [OTf]离子液体(ILs)进行循环伏安法(CV),以分析TEMPO衍生物的氧化还原电势。先前显示前者可通过与阳离子的质子转移反应淬灭TEMPO的氨氧基阴离子,而后者则支持TEMPO还原过程的不可逆性。在TEMPO衍生物的CV结果中,表明[C 4mpyr] [OTf]可以在4-氰基-TEMPO的还原中提供高度的可逆性,而在TEMPOL的还原中提供中等的可逆性。相比之下,在[C 2 mim] [BF 4 ]中4-氰基-TEMPO的还原主要是不可逆的,而TEMPOL显示出完全不可逆的。在任一测试的IL中,4-Oxo-TEMPO均未显示任何明显的还原可逆性。还原电位显示衍生物之间的变化很小,IL之间的变化为0.2 V,对于[C 4]中的TEMPOL,在-1.43 V vs. Fc / Fc +处观察到最大的负还原电位mpyr] [OTf]。为了解释还原过程可逆性的不同程度,使用高度相关的量子化学方法研究了涉及质子转移到氨氧基阴离子上的四种类型的副反应。已显示,从IL阳离子转移质子具有淬灭所有三个氨氧基阴离子的能力,具体取决于所用的IL。平均而言,TEMPOL被证明最容易从IL阳离子转移质子,平均吉布斯自由能(GFE)为10.5 kJ mol -1比4-氰基-TEMPO具有更大的负性,后者被证明具有最高的质子转移GFE。水和氨氧基阴离子之间的副反应也被认为有被示出为最具反应性的降解与水-12.6千焦mol的GFE有助于测试的氨氧基阴离子的降解,用4-氧代TEMPO的电位- 1。发现4-Oxo-TEMPO由于其氨氧基阴离子和自由基形式而具有高负质子转移GFE ,分别为-47.9 kJ mol -1和-57.7 kJ mol -1,因此非常易于自猝灭。总体而言,建议使用4-氰基-TEMPO作为用TEMPOL测试的最稳定的氨氧基阴离子,因此,如果将IL调整为使其稳定性最大化,则可以提供一种可行的替代方法来提高溶解度。
更新日期:2021-01-22
中文翻译:

TEMPO衍生物在离子液体中的电化学表征和热力学分析
在这项研究中,我们研究了三种TEMPO衍生物-TEMPOL,4-氰基-TEMPO和4-氧代-TEMPO的还原过程的可逆性。使用[C 2 mim] [BF 4 ]和[C 4 mpyr] [OTf]离子液体(ILs)进行循环伏安法(CV),以分析TEMPO衍生物的氧化还原电势。先前显示前者可通过与阳离子的质子转移反应淬灭TEMPO的氨氧基阴离子,而后者则支持TEMPO还原过程的不可逆性。在TEMPO衍生物的CV结果中,表明[C 4mpyr] [OTf]可以在4-氰基-TEMPO的还原中提供高度的可逆性,而在TEMPOL的还原中提供中等的可逆性。相比之下,在[C 2 mim] [BF 4 ]中4-氰基-TEMPO的还原主要是不可逆的,而TEMPOL显示出完全不可逆的。在任一测试的IL中,4-Oxo-TEMPO均未显示任何明显的还原可逆性。还原电位显示衍生物之间的变化很小,IL之间的变化为0.2 V,对于[C 4]中的TEMPOL,在-1.43 V vs. Fc / Fc +处观察到最大的负还原电位mpyr] [OTf]。为了解释还原过程可逆性的不同程度,使用高度相关的量子化学方法研究了涉及质子转移到氨氧基阴离子上的四种类型的副反应。已显示,从IL阳离子转移质子具有淬灭所有三个氨氧基阴离子的能力,具体取决于所用的IL。平均而言,TEMPOL被证明最容易从IL阳离子转移质子,平均吉布斯自由能(GFE)为10.5 kJ mol -1比4-氰基-TEMPO具有更大的负性,后者被证明具有最高的质子转移GFE。水和氨氧基阴离子之间的副反应也被认为有被示出为最具反应性的降解与水-12.6千焦mol的GFE有助于测试的氨氧基阴离子的降解,用4-氧代TEMPO的电位- 1。发现4-Oxo-TEMPO由于其氨氧基阴离子和自由基形式而具有高负质子转移GFE ,分别为-47.9 kJ mol -1和-57.7 kJ mol -1,因此非常易于自猝灭。总体而言,建议使用4-氰基-TEMPO作为用TEMPOL测试的最稳定的氨氧基阴离子,因此,如果将IL调整为使其稳定性最大化,则可以提供一种可行的替代方法来提高溶解度。


























 京公网安备 11010802027423号
京公网安备 11010802027423号