Journal of Industrial and Engineering Chemistry ( IF 6.1 ) Pub Date : 2021-01-22 , DOI: 10.1016/j.jiec.2021.01.016 Abu Saad Ansari , Jeong Woo Han , Bonggeun Shong
|
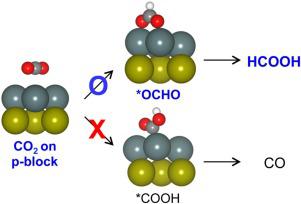
When used as electrocatalysts for the reduction of CO2, p-block metals (e.g., Bi, Sb, Tl, Pb, In, and Sn) are known to exhibit high selectivity toward the production of formic acid (HCOOH). Nonetheless, the current knowledge on the two key intermediates, namely  COOH and
COOH and  OCHO, is lacking, which hinders the mechanistic understanding and development of efficient p-block metal catalysts. In this study, the molecular adsorption phenomena related to the electrocatalytic reduction of CO2 (ERC) on p-block metal surfaces are investigated using dispersion-corrected density functional theory (DFT-D) calculations. It is demonstrated that the surfaces of p-block metals display low chemical reactivity toward CO2. The adsorbates of
OCHO, is lacking, which hinders the mechanistic understanding and development of efficient p-block metal catalysts. In this study, the molecular adsorption phenomena related to the electrocatalytic reduction of CO2 (ERC) on p-block metal surfaces are investigated using dispersion-corrected density functional theory (DFT-D) calculations. It is demonstrated that the surfaces of p-block metals display low chemical reactivity toward CO2. The adsorbates of  OCHO with O
OCHO with O metal bonds are consistently more stable than those of
metal bonds are consistently more stable than those of  COOH with C
COOH with C metal bonds. Consequently, the adsorption of
metal bonds. Consequently, the adsorption of  OCHO is favored over
OCHO is favored over  COOH adsorption, leading to the selective formation of HCOOH instead of CO. Structural, vibrational, and electronic properties of these two adsorbates are discussed to facilitate future experimental mechanistic studies.
COOH adsorption, leading to the selective formation of HCOOH instead of CO. Structural, vibrational, and electronic properties of these two adsorbates are discussed to facilitate future experimental mechanistic studies.
中文翻译:

催化还原p嵌段元素表面上的CO 2的中间体
已知当p-嵌段金属(例如Bi,Sb,Tl,Pb,In和Sn)用作还原CO 2的电催化剂时,对甲酸(HCOOH)的生产显示出高选择性。但是,目前缺乏关于两个关键中间体(即 COOH和
COOH和 OCHO)的知识,这阻碍了对有效的p嵌段金属催化剂的机械理解和开发。在这项研究中,使用分散校正的密度泛函理论(DFT-D)计算来研究与p嵌段金属表面上的CO 2(ERC)电催化还原有关的分子吸附现象。已经证明,p-嵌段金属的表面显示出对CO 2的低化学反应性。的吸附物
OCHO)的知识,这阻碍了对有效的p嵌段金属催化剂的机械理解和开发。在这项研究中,使用分散校正的密度泛函理论(DFT-D)计算来研究与p嵌段金属表面上的CO 2(ERC)电催化还原有关的分子吸附现象。已经证明,p-嵌段金属的表面显示出对CO 2的低化学反应性。的吸附物 具有O
具有O 金属键的OCHO始终比
金属键的OCHO始终比 具有C
具有C 金属键的COOH稳定。因此,
金属键的COOH稳定。因此, OCHO的吸附比
OCHO的吸附比 COOH的吸附更受青睐,从而导致选择性地形成HCOOH而不是CO。讨论了这两种吸附物的结构,振动和电子性质,以利于将来的实验机理研究。
COOH的吸附更受青睐,从而导致选择性地形成HCOOH而不是CO。讨论了这两种吸附物的结构,振动和电子性质,以利于将来的实验机理研究。



























 京公网安备 11010802027423号
京公网安备 11010802027423号