Chemosphere ( IF 8.8 ) Pub Date : 2021-01-22 , DOI: 10.1016/j.chemosphere.2021.129754 Ting Wang 1 , Taobo Huang 2 , Huan Jiang 2 , Ruoqi Ma 2
|
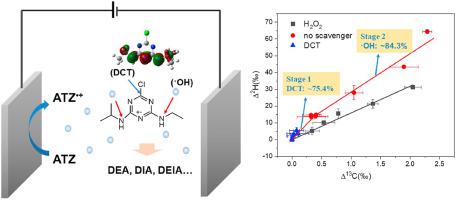
Direct charge transfer (DCT) and •OH attack played important roles in contaminant degradation by BDD electrochemical oxidation. Their separate contributions and potential bond-cleavage processes were required but lacking. Here, we carried out promising compound-specific isotope fractionation analysis (CSIA) to explore 13C and 2H isotope fractionation of atrazine (ATZ), followed by assessing the reaction pathway by BDD anode. The correlation of 2H and 13C fractionation allows to remarkably differentiate DCT process and •OH attack, with Ʌ values of 18.99 and 53.60, respectively. Radical quenching identified that •OH accounted for 79.0%∼88.5% in the whole reaction. While CSIA methods provided biased results, which suggested that ATZ degradation exhibited two stages with •OH contributions of 24.6% and 84.3% respectively, confirming CSIA was more sensitive and provided more possibilities to estimate degradation processes. Combined with Fukui index and intermediate products identification, we deduced that dechlorination-hydroxylation mainly occurred in the first 30 min by DCT reaction. While lateral chain oxidation with C‒N broken was the governing route once •OH was largely generated, with the production of DEA (m/z 188), DIA (m/z 174), DEIA (m/z 146) and DEIHA (m/z 128). Our results demonstrated that isotope fractionation can offer “isotopic footprints” for identifying the rate-limiting steps and bond breakage process, and opens new avenues for degradation pathways of contaminants.
中文翻译:

BDD 阳极对阿特拉津的电化学降解:化合物特异性稳定同位素分析和 DFT 模拟的证据
直接电荷转移 (DCT) 和 •OH 攻击在 BDD 电化学氧化的污染物降解中起着重要作用。他们的独立贡献和潜在的键断裂过程是必需的,但缺乏。在这里,我们进行了有前途的化合物特异性同位素分馏分析 (CSIA) 以探索阿特拉津 (ATZ) 的13 C 和2 H 同位素分馏,然后通过 BDD 阳极评估反应途径。2 H和13的相关性C 分馏允许显着区分 DCT 过程和 •OH 攻击,其 Ʌ 值分别为 18.99 和 53.60。自由基淬灭表明•OH占整个反应的79.0%~88.5%。虽然 CSIA 方法提供了有偏差的结果,这表明 ATZ 降解表现出两个阶段,•OH 贡献分别为 24.6% 和 84.3%,证实 CSIA 更敏感,并提供了更多估计降解过程的可能性。结合福井指数和中间产物鉴定,我们推断DCT反应主要发生在前30分钟的脱氯-羟基化反应。一旦大量产生•OH,侧链氧化和 C-N 断裂是主导途径,同时产生 DEA (m/z 188)、DIA (m/z 174)、DEIA (m/z 146) 和 DEIHA (质荷比 128)。


























 京公网安备 11010802027423号
京公网安备 11010802027423号