当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
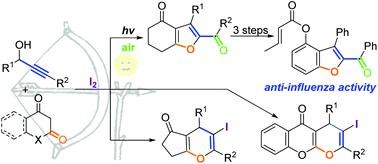
Regioselective synthesis of fused oxa-heterocycles via iodine-mediated annulation of cyclic 1,3-dicarbonyl compounds with propargylic alcohols
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2021-1-11 , DOI: 10.1039/d0qo01496f Liang Zhang 1, 2, 3, 4 , Neng-Jie Mou 1, 2, 3, 4 , Dong-Rong Xiao 1, 2, 3, 4 , Xin Zhuang 1, 2, 3, 4 , Xiao-Long Lin 1, 2, 3, 4 , Tian Cai 1, 2, 3, 4, 5 , Qun-Li Luo 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2021-1-11 , DOI: 10.1039/d0qo01496f Liang Zhang 1, 2, 3, 4 , Neng-Jie Mou 1, 2, 3, 4 , Dong-Rong Xiao 1, 2, 3, 4 , Xin Zhuang 1, 2, 3, 4 , Xiao-Long Lin 1, 2, 3, 4 , Tian Cai 1, 2, 3, 4, 5 , Qun-Li Luo 1, 2, 3, 4, 5
Affiliation
|
Functionalized oxa-heterocycles are basic structural units in various natural products and bioactive molecules. Synthetic methodologies to obtain fused oxa-heterocycles from propargylic precursors have received increasing interest. In particular, iodine-mediated cascade electrophilic cyclizations of propargylic compounds readily lead to numerous novel cyclic scaffolds. However, the oxygen atom of the aliphatic carbonyl acting as the nucleophile in the iodocyclization of alkynyl-tethered compounds has not been reported. Herein, we describe an efficient regioselective synthesis of fused oxa-heterocycles through iodine-mediated annulation of propargylic alcohols with cyclic 1,3-dicarbonyl compounds. Using this methodology, one-pot regioselective syntheses of 2-acylated dihydrobenzofuranones and pyrano[2,3-b]chromenones from propargylic alcohols were readily achieved.
中文翻译:

通过碘介导的环状1,3-二羰基化合物与炔丙醇的环选择性合成稠合的氧杂杂环
功能化的氧杂杂环是各种天然产物和生物活性分子中的基本结构单元。从炔丙基前体获得稠合的氧杂杂环的合成方法受到越来越多的关注。特别地,碘介导的炔丙基化合物的级联亲电环化容易产生许多新颖的环状支架。然而,尚未报道在炔基连接的化合物的碘环化中充当亲核试剂的脂族羰基的氧原子。在本文中,我们描述了通过碘介导的炔丙基醇与环状1,3-二羰基化合物的环合反应,稠合的氧杂杂环的有效区域选择性合成。使用这种方法,一锅的区域选择性合成2-酰化的二氢苯并呋喃酮和吡喃[2,3- b炔丙醇中的[]壬酮很容易实现。
更新日期:2021-01-21
中文翻译:

通过碘介导的环状1,3-二羰基化合物与炔丙醇的环选择性合成稠合的氧杂杂环
功能化的氧杂杂环是各种天然产物和生物活性分子中的基本结构单元。从炔丙基前体获得稠合的氧杂杂环的合成方法受到越来越多的关注。特别地,碘介导的炔丙基化合物的级联亲电环化容易产生许多新颖的环状支架。然而,尚未报道在炔基连接的化合物的碘环化中充当亲核试剂的脂族羰基的氧原子。在本文中,我们描述了通过碘介导的炔丙基醇与环状1,3-二羰基化合物的环合反应,稠合的氧杂杂环的有效区域选择性合成。使用这种方法,一锅的区域选择性合成2-酰化的二氢苯并呋喃酮和吡喃[2,3- b炔丙醇中的[]壬酮很容易实现。



























 京公网安备 11010802027423号
京公网安备 11010802027423号