当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ex vivo identification of circulating tumor cells in peripheral blood by fluorometric “turn on” aptamer nanoparticles
Chemical Science ( IF 8.4 ) Pub Date : 2020-12-22 , DOI: 10.1039/d0sc05112h Wenxi Xia 1 , Xiaoyan Shangguan 1 , Miao Li 1, 2 , Yang Wang 1 , Dongmei Xi 1 , Wen Sun 1 , Jiangli Fan 1 , Kun Shao 1 , Xiaojun Peng 1
Chemical Science ( IF 8.4 ) Pub Date : 2020-12-22 , DOI: 10.1039/d0sc05112h Wenxi Xia 1 , Xiaoyan Shangguan 1 , Miao Li 1, 2 , Yang Wang 1 , Dongmei Xi 1 , Wen Sun 1 , Jiangli Fan 1 , Kun Shao 1 , Xiaojun Peng 1
Affiliation
|
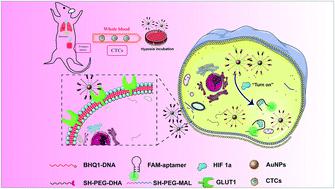
The detection of the circulating tumor cells (CTCs) detached from solid tumors has emerged as a burgeoning topic for cancer diagnosis and treatment. The conventional CTC enrichment and identification mainly rely on the specific binding of the antibodies on the capture interface of the magnetic nanoparticles with the corresponding biomarkers on the cell membranes. However, these methods could easily generate false-negative results due to the extremely low concentration of CTCs and the internal heterogeneity of the tumor cells. Herein, with the aim of selectively identifying CTCs and improving the detection accuracy in peripheral blood, we designed the fluorometric “turn on” Au nanoparticles (DHANs) with the modification of a tumor-targeted moiety, dehydroascorbic acid (DHA) and a fluorometric aptamer, which could be “switched-on” by an over-expressed intracellular protein, namely hypoxia-inducible factor-1α (HIF 1α). This novel nanoformulated detection platform demonstrated the great capacity for visualizing various CTCs in peripheral blood with significantly improved detection efficiency and sensitivity. As a result, the nanoplatform has a great potential to be further applied for CTC detection in vitro or in vivo, which holds promise for extensive CTC studies.
中文翻译:

通过荧光“开启”适体纳米粒子的离体鉴定外周血中循环肿瘤细胞
与实体瘤分离的循环肿瘤细胞(CTC)的检测已成为癌症诊断和治疗的新兴主题。常规的CTC富集和鉴定主要依赖于磁性纳米颗粒的捕获界面上的抗体与细胞膜上相应的生物标记物的特异性结合。但是,由于CTC的浓度极低且肿瘤细胞内部异质性,这些方法很容易产生假阴性结果。在本文中,为了选择性地识别四氯化碳并提高外周血中的检测准确性,我们设计了带有肿瘤靶向部分,脱氢抗坏血酸(DHA)和荧光适体的荧光“开启”金纳米颗粒(DHAN)。 ,它可能被过表达的细胞内蛋白即缺氧诱导因子-1α(HIF1α)“打开”。这种新颖的纳米级检测平台展示了显着提高检测效率和灵敏度的可视化外周血中各种CTC的强大能力。结果,纳米平台具有巨大的潜力,可进一步用于CTC检测体外或体内,这为广泛的CTC研究提供了希望。
更新日期:2021-01-21
中文翻译:

通过荧光“开启”适体纳米粒子的离体鉴定外周血中循环肿瘤细胞
与实体瘤分离的循环肿瘤细胞(CTC)的检测已成为癌症诊断和治疗的新兴主题。常规的CTC富集和鉴定主要依赖于磁性纳米颗粒的捕获界面上的抗体与细胞膜上相应的生物标记物的特异性结合。但是,由于CTC的浓度极低且肿瘤细胞内部异质性,这些方法很容易产生假阴性结果。在本文中,为了选择性地识别四氯化碳并提高外周血中的检测准确性,我们设计了带有肿瘤靶向部分,脱氢抗坏血酸(DHA)和荧光适体的荧光“开启”金纳米颗粒(DHAN)。 ,它可能被过表达的细胞内蛋白即缺氧诱导因子-1α(HIF1α)“打开”。这种新颖的纳米级检测平台展示了显着提高检测效率和灵敏度的可视化外周血中各种CTC的强大能力。结果,纳米平台具有巨大的潜力,可进一步用于CTC检测体外或体内,这为广泛的CTC研究提供了希望。


























 京公网安备 11010802027423号
京公网安备 11010802027423号