当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
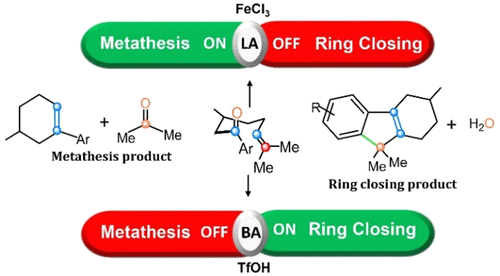
Brønsted-Acid-Catalyzed Intramolecular Carbonyl–Olefin Reactions: Interrupted Metathesis vs Carbonyl-Ene Reaction
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-01-21 , DOI: 10.1021/acs.joc.0c03021 Tanmay Malakar 1 , Paul M Zimmerman 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-01-21 , DOI: 10.1021/acs.joc.0c03021 Tanmay Malakar 1 , Paul M Zimmerman 1
Affiliation
|
Lewis acid catalysts have been shown to promote carbonyl–olefin metathesis through a critical four-membered-ring oxetane intermediate. Recently, Brønsted-acid catalysis of related substrates was similarly proposed to result in a transient oxetane, which fragments within a single elementary step via a postulated oxygen-atom transfer mechanism. Herein, careful quantum chemical investigations show that Brønsted acid (triflic acid, TfOH) instead invokes a mechanistic switch to a carbonyl-ene reaction, and oxygen-atom transfer is uncompetitive. TfOH’s conjugate base is also found to rearrange H atoms and allow isomerization of the carbocations that appear after the carbonyl-ene reaction. The mechanism explains available experimental information, including the skipped diene species that appear transiently before product formation. The present study clarifies the mechanism for activation of intramolecular carbonyl–olefin substrates by Brønsted acids and provides important insights that will help develop this exciting class of catalysts.
中文翻译:

布朗斯台德酸催化的分子内羰基-烯烃反应:中断复分解与羰基-烯反应
路易斯酸催化剂已被证明可通过关键的四元环氧杂环丁烷中间体促进羰基-烯烃复分解。最近,类似地提出了相关底物的布朗斯台德酸催化产生瞬态氧杂环丁烷,其通过假定的氧原子转移机制在单个基本步骤内断裂。在这里,仔细的量子化学研究表明,布朗斯台德酸(三氟甲磺酸,TfOH)反而引发了对羰基-烯反应的机械转换,并且氧原子转移是没有竞争力的。还发现 TfOH 的共轭碱可重新排列 H 原子并允许在羰基-烯反应后出现的碳阳离子异构化。该机制解释了可用的实验信息,包括在产品形成之前短暂出现的跳过的二烯物种。
更新日期:2021-02-05
中文翻译:

布朗斯台德酸催化的分子内羰基-烯烃反应:中断复分解与羰基-烯反应
路易斯酸催化剂已被证明可通过关键的四元环氧杂环丁烷中间体促进羰基-烯烃复分解。最近,类似地提出了相关底物的布朗斯台德酸催化产生瞬态氧杂环丁烷,其通过假定的氧原子转移机制在单个基本步骤内断裂。在这里,仔细的量子化学研究表明,布朗斯台德酸(三氟甲磺酸,TfOH)反而引发了对羰基-烯反应的机械转换,并且氧原子转移是没有竞争力的。还发现 TfOH 的共轭碱可重新排列 H 原子并允许在羰基-烯反应后出现的碳阳离子异构化。该机制解释了可用的实验信息,包括在产品形成之前短暂出现的跳过的二烯物种。

























 京公网安备 11010802027423号
京公网安备 11010802027423号