当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Recovery of N2O: Energy-Efficient and Structure-Driven Clathrate-Based Greenhouse Gas Separation
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2021-01-21 , DOI: 10.1021/acs.est.0c06233 Jiyeong Jang 1 , Sol Geo Lim 1 , Jae Hak Jeong 2 , Appu Vengattoor Raghu 2 , Jong-Won Lee 3 , Minjun Cha 4 , Sanehiro Muromachi 5 , Yoshitaka Yamamoto 5 , Ji-Ho Yoon 1, 2, 5
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2021-01-21 , DOI: 10.1021/acs.est.0c06233 Jiyeong Jang 1 , Sol Geo Lim 1 , Jae Hak Jeong 2 , Appu Vengattoor Raghu 2 , Jong-Won Lee 3 , Minjun Cha 4 , Sanehiro Muromachi 5 , Yoshitaka Yamamoto 5 , Ji-Ho Yoon 1, 2, 5
Affiliation
|
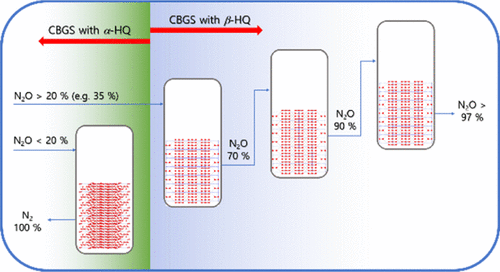
N2O has 300 times more global warming potential than CO2 and is also one of the main stratospheric ozone-depleting substances emitted by human activities such as agriculture, industry, and the combustion of fossil fuels and solid waste. We present here an energy-efficient clathrate-based greenhouse gas-separation (CBGS) technology that can operate at room temperature for selectively recovering N2O from gas mixtures. Clathrate formation between α-form/β-form hydroquinone (α-HQ/β-HQ) and gas mixtures reveals guest-specific and structure-driven selectivity, revealing the preferential capture of N2O in β-HQ and the molecular sieving characteristics of α-HQ. With a maximum gas storage capacity and cage occupancy of 54.1 cm3 g–1 and 0.86, respectively, HQ clathrate compounds including N2O are stable at room temperature and atmospheric pressure and thus can be easily synthesized, treated, and recycled via commercial CBGS processes. High selectivity for N2O recovery was observed during β-HQ clathrate formation from N2O/N2 gas mixtures with N2O concentrations exceeding 20%, whereas α-HQ traps only N2 molecules from gas mixtures. Full characterization using X-ray diffraction, scanning electron microscopy, Raman spectroscopy, solid-state nuclear magnetic resonance, and compositional analysis and the formation kinetics of HQ clathrates was conducted to verify the peculiar selectivity behavior and to design the conceptual CBGS process. These results provide a new playground on which to tailor host–guest materials and develop commercial processes for the recovery and/or sequestration of greenhouse gases.
中文翻译:

N 2 O的回收:节能和结构驱动的基于包合物的温室气体分离
N 2 O具有比CO 2高300倍的全球变暖潜能,并且也是人类活动(如农业,工业以及化石燃料和固体废物的燃烧)排放的主要平流层臭氧消耗物质之一。我们在这里提出的是可以在室温下,用于选择性地回收正操作的能量高效的基于包合物温室气体分离(CBGS)技术2从气体混合物中O操作。α-形式/β-形式对苯二酚(α-HQ/β-HQ)与气体混合物之间的包合物形成显示出客体特异性和结构驱动的选择性,揭示了β-HQ中N 2 O的优先捕获和分子筛特性α-HQ。最大储气量和笼式占用率为54.1 cm 3分别为g –1和0.86的HQ笼形化合物(包括N 2 O)在室温和大气压下稳定,因此可以通过商业CBGS工艺轻松合成,处理和回收。在N 2 O浓度超过20%的N 2 O / N 2混合气体中形成β-HQ包合物期间,观察到了对N 2 O回收的高选择性,而α-HQ仅捕获了N 2气体混合物中的分子。使用X射线衍射,扫描电子显微镜,拉曼光谱,固态核磁共振以及成分分析和HQ包合物的形成动力学进行了全面表征,以验证特殊的选择性行为并设计概念性CBGS工艺。这些结果提供了一个新的场所,可以在此基础上定制接待宾客的材料并开发用于回收和/或隔离温室气体的商业流程。
更新日期:2021-03-16
中文翻译:

N 2 O的回收:节能和结构驱动的基于包合物的温室气体分离
N 2 O具有比CO 2高300倍的全球变暖潜能,并且也是人类活动(如农业,工业以及化石燃料和固体废物的燃烧)排放的主要平流层臭氧消耗物质之一。我们在这里提出的是可以在室温下,用于选择性地回收正操作的能量高效的基于包合物温室气体分离(CBGS)技术2从气体混合物中O操作。α-形式/β-形式对苯二酚(α-HQ/β-HQ)与气体混合物之间的包合物形成显示出客体特异性和结构驱动的选择性,揭示了β-HQ中N 2 O的优先捕获和分子筛特性α-HQ。最大储气量和笼式占用率为54.1 cm 3分别为g –1和0.86的HQ笼形化合物(包括N 2 O)在室温和大气压下稳定,因此可以通过商业CBGS工艺轻松合成,处理和回收。在N 2 O浓度超过20%的N 2 O / N 2混合气体中形成β-HQ包合物期间,观察到了对N 2 O回收的高选择性,而α-HQ仅捕获了N 2气体混合物中的分子。使用X射线衍射,扫描电子显微镜,拉曼光谱,固态核磁共振以及成分分析和HQ包合物的形成动力学进行了全面表征,以验证特殊的选择性行为并设计概念性CBGS工艺。这些结果提供了一个新的场所,可以在此基础上定制接待宾客的材料并开发用于回收和/或隔离温室气体的商业流程。



























 京公网安备 11010802027423号
京公网安备 11010802027423号