当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydrolytic Degradation of Heparin in Acidic Environments: Nuclear Magnetic Resonance Reveals Details of Selective Desulfation
ACS Applied Materials & Interfaces ( IF 9.5 ) Pub Date : 2021-01-20 , DOI: 10.1021/acsami.0c20198 Aleksandra M. Kozlowski 1 , Edwin A. Yates 2 , Johannes P. Roubroeks 3 , Kristoffer Tømmeraas 3 , Alan M. Smith 1 , Gordon A. Morris 1
ACS Applied Materials & Interfaces ( IF 9.5 ) Pub Date : 2021-01-20 , DOI: 10.1021/acsami.0c20198 Aleksandra M. Kozlowski 1 , Edwin A. Yates 2 , Johannes P. Roubroeks 3 , Kristoffer Tømmeraas 3 , Alan M. Smith 1 , Gordon A. Morris 1
Affiliation
|
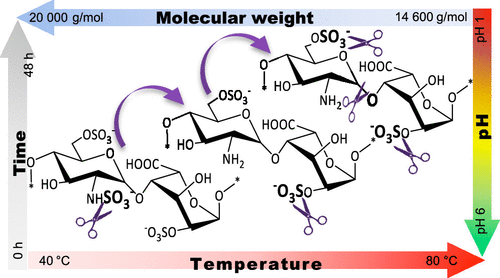
Heparin is a complex glycosaminoglycan, derived mainly from pig mucosa, used therapeutically for its anticoagulant activity. Yet, owing largely to the chain complexity, the progressive effects of environmental conditions on heparin structure have not been fully described. A systematic study of the influence of acidic hydrolysis on heparin chain length and substitution has therefore been conducted. Changes in the sulfation pattern, monitored via 2D NMR, revealed initial de-N-sulfation of the molecule (pH 1/ 40 °C) and unexpectedly identified the secondary sulfate of iduronate as more labile than the 6-O-sulfate of glucosamine residues under these conditions (pH 1/ 60 °C). Additionally, the loss of sulfate groups, rather than depolymerization, accounted for most of the reduction in molecular weight. This provides an alternative route to producing partially 2-O-de-sulfated heparin derivatives that avoids using conventional basic conditions and may be of value in the optimization of processes associated with the production of heparin pharmaceuticals.
中文翻译:

酸性环境中肝素的水解降解:核磁共振揭示了选择性脱硫的细节
肝素是一种复杂的糖胺聚糖,主要衍生自猪粘膜,在治疗上因其抗凝活性而使用。然而,主要由于链的复杂性,尚未充分描述环境条件对肝素结构的逐步影响。因此,已经进行了酸性水解对肝素链长和取代的影响的系统研究。硫酸化模式的变化,通过2D NMR揭示了分子的初始脱N-硫酸盐(pH 1/40°C),并出乎意料地确定了在这些条件下(pH 1/60)葡萄糖酸残基的6-O-硫酸盐比二氨基葡萄糖的不稳定。 °C)。另外,硫酸根基团的损失而不是解聚是造成分子量降低的主要原因。这为生产部分2-O-脱硫的肝素衍生物提供了另一种途径,该途径避免了使用常规基本条件,并且可能在优化与肝素药物生产相关的过程中具有价值。
更新日期:2021-02-03
中文翻译:

酸性环境中肝素的水解降解:核磁共振揭示了选择性脱硫的细节
肝素是一种复杂的糖胺聚糖,主要衍生自猪粘膜,在治疗上因其抗凝活性而使用。然而,主要由于链的复杂性,尚未充分描述环境条件对肝素结构的逐步影响。因此,已经进行了酸性水解对肝素链长和取代的影响的系统研究。硫酸化模式的变化,通过2D NMR揭示了分子的初始脱N-硫酸盐(pH 1/40°C),并出乎意料地确定了在这些条件下(pH 1/60)葡萄糖酸残基的6-O-硫酸盐比二氨基葡萄糖的不稳定。 °C)。另外,硫酸根基团的损失而不是解聚是造成分子量降低的主要原因。这为生产部分2-O-脱硫的肝素衍生物提供了另一种途径,该途径避免了使用常规基本条件,并且可能在优化与肝素药物生产相关的过程中具有价值。


























 京公网安备 11010802027423号
京公网安备 11010802027423号