Cell ( IF 64.5 ) Pub Date : 2021-01-21 , DOI: 10.1016/j.cell.2020.12.025 Maya Maor-Nof 1 , Zohar Shipony 1 , Rodrigo Lopez-Gonzalez 2 , Lisa Nakayama 1 , Yong-Jie Zhang 3 , Julien Couthouis 1 , Jacob A Blum 1 , Patricia A Castruita 4 , Gabriel R Linares 5 , Kai Ruan 6 , Gokul Ramaswami 7 , David J Simon 8 , Aviv Nof 1 , Manuel Santana 5 , Kyuho Han 1 , Nasa Sinnott-Armstrong 1 , Michael C Bassik 1 , Daniel H Geschwind 7 , Marc Tessier-Lavigne 8 , Laura D Attardi 9 , Thomas E Lloyd 6 , Justin K Ichida 5 , Fen-Biao Gao 2 , William J Greenleaf 1 , Jennifer S Yokoyama 4 , Leonard Petrucelli 3 , Aaron D Gitler 1
|
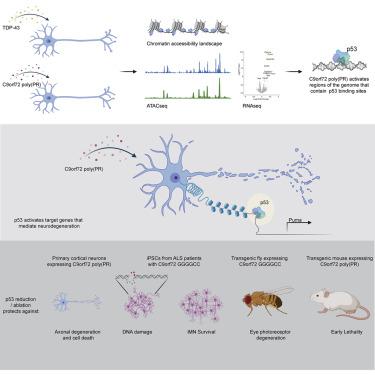
The most common genetic cause of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) is a GGGGCC repeat expansion in the C9orf72 gene. We developed a platform to interrogate the chromatin accessibility landscape and transcriptional program within neurons during degeneration. We provide evidence that neurons expressing the dipeptide repeat protein poly(proline-arginine), translated from the C9orf72 repeat expansion, activate a highly specific transcriptional program, exemplified by a single transcription factor, p53. Ablating p53 in mice completely rescued neurons from degeneration and markedly increased survival in a C9orf72 mouse model. p53 reduction also rescued axonal degeneration caused by poly(glycine-arginine), increased survival of C9orf72 ALS/FTD-patient-induced pluripotent stem cell (iPSC)-derived motor neurons, and mitigated neurodegeneration in a C9orf72 fly model. We show that p53 activates a downstream transcriptional program, including Puma, which drives neurodegeneration. These data demonstrate a neurodegenerative mechanism dynamically regulated through transcription-factor-binding events and provide a framework to apply chromatin accessibility and transcription program profiles to neurodegeneration.
中文翻译:

p53 是驱动由 C9orf72 poly(PR) 引起的神经变性的中枢调节因子
肌萎缩侧索硬化症 (ALS) 和额颞叶痴呆 (FTD) 最常见的遗传原因是 C9orf72 基因中的 GGGGCC 重复扩增。我们开发了一个平台来询问退化过程中神经元内的染色质可及性景观和转录程序。我们提供的证据表明神经元表达从C9orf72重复扩增翻译而来的二肽重复蛋白 poly(脯氨酸-精氨酸),激活了一个高度特异性的转录程序,例如单个转录因子 p53。在C9orf72小鼠模型中,消融小鼠中的 p53 完全挽救了神经元的退化,并显着提高了存活率。p53 减少还挽救了由聚(甘氨酸 - 精氨酸)引起的轴突变性,增加了C9orf72 ALS/FTD 患者诱导的多能干细胞 (iPSC) 衍生的运动神经元,并减轻了C9orf72飞行模型中的神经变性。我们表明 p53 激活了一个下游转录程序,包括Puma,它驱动神经退行性变。这些数据证明了通过转录因子结合事件动态调节的神经退行性机制,并提供了将染色质可及性和转录程序配置文件应用于神经退行性变的框架。

























 京公网安备 11010802027423号
京公网安备 11010802027423号