Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrocatalytically inactive copper improves the water adsorption/dissociation on Ni3S2 for accelerated alkaline and neutral hydrogen evolution
Nanoscale ( IF 6.7 ) Pub Date : 2021-1-6 , DOI: 10.1039/d0nr07275c Lei Zhang 1, 2, 3, 4 , Xiaorui Gao 1, 2, 3, 4 , Ying Zhu 1, 2, 3, 4 , Acan Liu 1, 2, 3, 4 , Huilong Dong 1, 2, 3, 4 , Dajun Wu 1, 2, 3, 4 , Zhida Han 1, 2, 3, 4 , Wei Wang 4, 5, 6, 7 , Yong Fang 1, 2, 3, 4 , Jie Zhang 1, 2, 3, 4 , Zongkui Kou 8, 9, 10, 11 , Bin Qian 1, 2, 3, 4 , Ting-Ting Wang 12, 13, 14, 15, 16
Nanoscale ( IF 6.7 ) Pub Date : 2021-1-6 , DOI: 10.1039/d0nr07275c Lei Zhang 1, 2, 3, 4 , Xiaorui Gao 1, 2, 3, 4 , Ying Zhu 1, 2, 3, 4 , Acan Liu 1, 2, 3, 4 , Huilong Dong 1, 2, 3, 4 , Dajun Wu 1, 2, 3, 4 , Zhida Han 1, 2, 3, 4 , Wei Wang 4, 5, 6, 7 , Yong Fang 1, 2, 3, 4 , Jie Zhang 1, 2, 3, 4 , Zongkui Kou 8, 9, 10, 11 , Bin Qian 1, 2, 3, 4 , Ting-Ting Wang 12, 13, 14, 15, 16
Affiliation
|
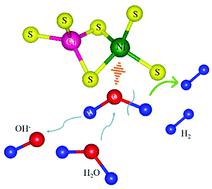
Nickel dichalcogenides, especially Ni3S2, present inferior alkaline and neutral hydrogen evolution activity due to their sluggish water dissociation kinetics. Although these materials hold promise as non-noble metal-based electrocatalysts for the hydrogen evolution reaction (HER) in acidic media, developing efficient strategies to enhance the water dissociation processes of nickel dichalcogenides in alkaline and neutral solutions is also an important area of research. The present work discloses an electrocatalytically inactive copper doping strategy to promote the water adsorption and dissociation process of Ni3S2 (Cu-Ni3S2) nanoparticles supported on nickel foam (NF) towards improving the alkaline and neutral hydrogen evolution reactions. Based on combined density functional theory calculations and electrochemical characterizations, the doping of Cu can accelerate the Volmer step and therefore strengthen the water adsorption/dissociation on the respective Ni sites and S sites during the HER process. As a result, the electrocatalyst exhibits superior and stable HER performance in both 1 M KOH and 1 M phosphate-buffered saline (PBS) solutions, with much lower overpotentials of 121 and 228 mV at a current density of 10 mA cm−2, respectively, in comparison to bare Ni3S2. We therefore conclude that the tailored control of the water adsorption/dissociation capability of Ni3S2 will open significant opportunities for the rational design of alkaline and neutral electrocatalysts from earth-abundant and stable materials.
中文翻译:

电催化惰性铜改善了Ni3S2上的水吸附/离解,促进了碱性和中性氢的释放
二卤化镍,尤其是Ni 3 S 2,由于其缓慢的水离解动力学而呈现出较弱的碱性和中性氢放出活性。尽管这些材料有望在酸性介质中用作氢生成反应(HER)的非贵金属基电催化剂,但开发有效的策略来增强二卤化镍在碱性和中性溶液中的水离解过程也是重要的研究领域。本工作公开了一种电催化惰性铜掺杂策略,以促进Ni 3 S 2(Cu-Ni 3 S 2)的水吸附和解离过程。)负载在泡沫镍(NF)上的纳米颗粒,可改善碱性和中性氢的释放反应。基于组合的密度泛函理论计算和电化学特性,Cu的掺杂可以加速Volmer步骤,从而在HER过程中加强各个Ni位和S位上的水吸附/离解。其结果是,电催化剂以10mA cm 2的电流密度显示出优异且稳定的HER在两者的1M KOH和1M磷酸盐缓冲盐水性能(PBS)溶液,用121和228毫伏的低得多的超电势-2分别,与裸露的Ni 3 S 2相比。因此,我们得出结论,镍的水吸附/解离能力的量身定制控制3 S 2将为从富含地球的稳定材料中合理设计碱性和中性电催化剂提供重要的机会。
更新日期:2021-01-20
中文翻译:

电催化惰性铜改善了Ni3S2上的水吸附/离解,促进了碱性和中性氢的释放
二卤化镍,尤其是Ni 3 S 2,由于其缓慢的水离解动力学而呈现出较弱的碱性和中性氢放出活性。尽管这些材料有望在酸性介质中用作氢生成反应(HER)的非贵金属基电催化剂,但开发有效的策略来增强二卤化镍在碱性和中性溶液中的水离解过程也是重要的研究领域。本工作公开了一种电催化惰性铜掺杂策略,以促进Ni 3 S 2(Cu-Ni 3 S 2)的水吸附和解离过程。)负载在泡沫镍(NF)上的纳米颗粒,可改善碱性和中性氢的释放反应。基于组合的密度泛函理论计算和电化学特性,Cu的掺杂可以加速Volmer步骤,从而在HER过程中加强各个Ni位和S位上的水吸附/离解。其结果是,电催化剂以10mA cm 2的电流密度显示出优异且稳定的HER在两者的1M KOH和1M磷酸盐缓冲盐水性能(PBS)溶液,用121和228毫伏的低得多的超电势-2分别,与裸露的Ni 3 S 2相比。因此,我们得出结论,镍的水吸附/解离能力的量身定制控制3 S 2将为从富含地球的稳定材料中合理设计碱性和中性电催化剂提供重要的机会。

























 京公网安备 11010802027423号
京公网安备 11010802027423号