当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Lean NOx Capture and Reduction by NH3via NO+ Intermediates over H-CHA at Room Temperature
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2021-01-19 , DOI: 10.1021/acs.jpcc.0c10913 Shunsaku Yasumura 1 , Chong Liu 1 , Takashi Toyao 1, 2 , Zen Maeno 1 , Ken-ichi Shimizu 1, 2
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2021-01-19 , DOI: 10.1021/acs.jpcc.0c10913 Shunsaku Yasumura 1 , Chong Liu 1 , Takashi Toyao 1, 2 , Zen Maeno 1 , Ken-ichi Shimizu 1, 2
Affiliation
|
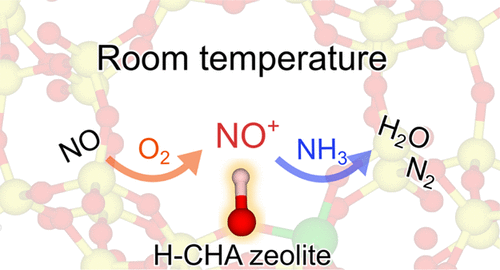
The oxidation of NO to NO2 and the subsequent reduction by NH3via a NO+ intermediate over a proton-type chabazite zeolite (H-CHA) were investigated by the combination of in situ infrared (IR) spectroscopy and density functional theory (DFT) calculations. The in situ IR spectral results indicate that the NO+ species formed under a flow of NO + O2 at 27–250 °C are more stable at lower temperatures over both H-CHA and copper-cation-exchanged CHA zeolite (Cu-CHA). The Arrhenius plot (T = 27–120 °C) shows a negative apparent activation barrier energy (−11.5 kJ mol–1) for the formation of NO+ species under the NO + O2 flow over H-CHA. The time course of the IR spectra at 27 °C shows that NO is oxidized by O2 to NO2 and then further converted via N2O4 to NO+ and NO3–. The subsequent exposure to NH3 at 27 °C reduces the NO+ species to N2. DFT calculations revealed that Brønsted acid sites in zeolite pores promote the dissociation of N2O4 intermediates into NO+ and NO3– species with a low activation barrier (15 kJ mol–1). Moreover, the computed activation barrier for the reduction of NO+ species by NH3 was considerably low (6 kJ mol–1). The experimental and theoretical results of this study demonstrate the high potential of Cu-free H-CHA zeolites for promoting lean NOx capture to form NO+ species and the subsequent reduction by NH3 at room temperature.
中文翻译:

贫NO X捕捉和还原NH 3经由NO +中间体较H-CHA在室温
结合原位红外光谱和密度泛函理论(DFT ),研究了质子型菱沸石(H-CHA)上NO氧化为NO 2以及随后通过NO +中间体被NH 3还原的过程。)计算。该原位IR光谱结果表明,NO + NO + O流下形成物种2在27-250℃下更稳定的在较低温度下在两个H-CHA和铜阳离子交换的沸石CHA(CU-CHA )。阿累尼乌斯图(T = 27–120°C)显示了形成NO的负表观活化势垒能(−11.5 kJ mol –1)NO + O 2下的+物质流过H-CHA。在27℃下示出了IR光谱的时间过程的NO被O氧化2至NO 2,然后进一步转化经由Ñ 2 Ò 4为NO +与NO 3 - 。随后在27°C下暴露于NH 3中,会将NO +物种还原为N 2。DFT计算表明,在沸石孔布朗斯台德酸位点促进N的离解2 ö 4个中间体成NO +与NO 3 -具有低激活势垒(15 kJ mol –1)的物种。此外,计算得出的通过NH 3还原NO +物种的活化势垒非常低(6 kJ mol –1)。本研究的实验和理论结果表明用于促进贫NO铜-自由H-CHA沸石的高电位X捕获以形成NO +物种和由NH随后的还原3在室温下。
更新日期:2021-01-28
中文翻译:

贫NO X捕捉和还原NH 3经由NO +中间体较H-CHA在室温
结合原位红外光谱和密度泛函理论(DFT ),研究了质子型菱沸石(H-CHA)上NO氧化为NO 2以及随后通过NO +中间体被NH 3还原的过程。)计算。该原位IR光谱结果表明,NO + NO + O流下形成物种2在27-250℃下更稳定的在较低温度下在两个H-CHA和铜阳离子交换的沸石CHA(CU-CHA )。阿累尼乌斯图(T = 27–120°C)显示了形成NO的负表观活化势垒能(−11.5 kJ mol –1)NO + O 2下的+物质流过H-CHA。在27℃下示出了IR光谱的时间过程的NO被O氧化2至NO 2,然后进一步转化经由Ñ 2 Ò 4为NO +与NO 3 - 。随后在27°C下暴露于NH 3中,会将NO +物种还原为N 2。DFT计算表明,在沸石孔布朗斯台德酸位点促进N的离解2 ö 4个中间体成NO +与NO 3 -具有低激活势垒(15 kJ mol –1)的物种。此外,计算得出的通过NH 3还原NO +物种的活化势垒非常低(6 kJ mol –1)。本研究的实验和理论结果表明用于促进贫NO铜-自由H-CHA沸石的高电位X捕获以形成NO +物种和由NH随后的还原3在室温下。


























 京公网安备 11010802027423号
京公网安备 11010802027423号