当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantioselective Total Synthesis of Nitraria Alkaloids: (+)-Nitramine, (+)-Isonitramine, (−)-Isonitramine, and (−)-Sibirine via Asymmetric Phase-Transfer Catalytic α-Allylations of α-Carboxylactams
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-01-19 , DOI: 10.1021/acs.joc.0c02573 Jewon Yang 1 , Yohan Park 2 , Sehun Yang 1 , Geumwoo Lee 1 , Min Woo Ha 3 , Mi-Hyun Kim 4 , Suckchang Hong 1 , Hyeung-Geun Park 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-01-19 , DOI: 10.1021/acs.joc.0c02573 Jewon Yang 1 , Yohan Park 2 , Sehun Yang 1 , Geumwoo Lee 1 , Min Woo Ha 3 , Mi-Hyun Kim 4 , Suckchang Hong 1 , Hyeung-Geun Park 1
Affiliation
|
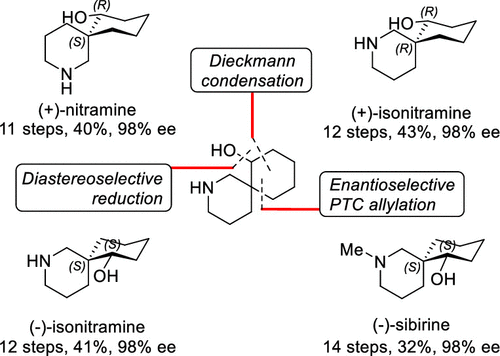
Many optically active 2-azaspirocyclic structures have frequently been found in biologically active natural products. In particular, Nitraria alkaloids, (+)-nitramine, (+)-isonitramine, (−)-isonitramine, and (−)-sibirine, have stereogenicity on their quaternary carbon of the 2-azaspiro[5,5]undecane-7-ol structure. To synthesize Nitraria alkaloids, we developed a new enantioselective synthetic method for chiral α-quaternary lactams via the α-alkylation of α-tert-butoxycarbonyl lactams. α-Alkylation of α-tert-butoxycarboxylactams in the circumstances of phase-transfer catalytic (PTC) system (solid KOH, toluene, and −40 °C) by virtue of the catalytic action of (S,S)-NAS bromide (5 mol %) furnished the corresponding α-alkyl-α-tert-butoxycarbonyl lactams in very high chemical (<99%) and enantioselectivity (<98% ee). Our catalytic methodology was successfully applied for the enantioselective total synthesis of Nitraria alkaloids. (+)-Isonitramine was obtained in 12 steps (98% ee, 43% yield) from δ-valerolactam through enantioselective phase-transfer catalytic allylation, Dieckmann condensation, and diastereoselective reduction as the key reactions. (−)-Sibirine and (+)-nitramine were prepared from (−)-isonitramine or its intermediate. Switching the phase-transfer catalyst from (S,S)-NAS bromide to (R,R)-NAS bromide afforded (−)-isonitramine (98% ee, 41% yield). (−)-Sibirine was synthesized by N-ethoxycarbonylation of (−)-isonitramine followed by reduction (98% ee, 14 steps, 32% yield). Furthermore, the diastereoselective reduction of (R)-2-benzhydryl-2-azaspiro[5.5]undecane-1,7-dione [(R)-15] followed by reductive removal of the diphenylmethyl group successfully gave (+)-nitramine (98% ee, 11 steps, 40% yield).
中文翻译:

对硝基生物碱的对映选择性全合成:α-羧基内酰胺的不对称相转移催化α-烯丙基化的(+)-硝胺,(+)-艾尼曲明,(-)-艾尼曲明和(-)-西比林
在生物活性的天然产物中经常发现许多旋光的2-氮杂螺环结构。尤其是,Nitraria生物碱,(+)-硝胺,(+)-异烟酰胺,(-)-异烟酰胺和(-)-西比林碱在其2-azaspiro [5,5]十一烷-7的季碳上具有立体异构性。 -ol结构。为了合成Nitraria生物碱,我们通过α-叔丁氧羰基内酰胺的α-烷基化开发了一种新的对映选择性合成方法,用于手性α-季内酰胺。α-α-烷基化叔-butoxycarboxylactams在相转移催化(PTC)系统的情况(固体KOH,甲苯,-40℃)凭借(催化作用的小号,小号)-NAS溴化物(5 mol%)以非常高的化学性质(<99%)和对映选择性(<98%ee)提供了相应的α-烷基-α-叔丁氧羰基内酰胺。我们的催化方法已成功地用于白背生物碱的对映选择性全合成。通过对映选择性相转移催化烯丙基化,Dieckmann缩合和非对映选择性还原作为关键反应,从δ-戊内酰胺分12步(98%ee,43%收率)获得(+)-艾尼曲明。(-)-西比林和(+)-硝胺由(-)-异硝胺或其中间体制备。将相转移催化剂从(S,S)-NAS溴化物转换为(R,R)-NAS溴化物提供(-)-异烟酰胺(98%ee,41%产率)。(-)-Sibirine是通过(-)-异硝胺的N-乙氧羰基化反应,然后还原反应合成的(98%ee,14步,收率32%)。此外,(R)-2-苯甲酰基-2-氮杂螺[5.5]十一烷-1,7-二酮[(R)-15 ]的非对映选择性还原,然后还原性去除二苯甲基,成功得到(+)-硝胺( ee:98%,11步,收率40%)。
更新日期:2021-03-19
中文翻译:

对硝基生物碱的对映选择性全合成:α-羧基内酰胺的不对称相转移催化α-烯丙基化的(+)-硝胺,(+)-艾尼曲明,(-)-艾尼曲明和(-)-西比林
在生物活性的天然产物中经常发现许多旋光的2-氮杂螺环结构。尤其是,Nitraria生物碱,(+)-硝胺,(+)-异烟酰胺,(-)-异烟酰胺和(-)-西比林碱在其2-azaspiro [5,5]十一烷-7的季碳上具有立体异构性。 -ol结构。为了合成Nitraria生物碱,我们通过α-叔丁氧羰基内酰胺的α-烷基化开发了一种新的对映选择性合成方法,用于手性α-季内酰胺。α-α-烷基化叔-butoxycarboxylactams在相转移催化(PTC)系统的情况(固体KOH,甲苯,-40℃)凭借(催化作用的小号,小号)-NAS溴化物(5 mol%)以非常高的化学性质(<99%)和对映选择性(<98%ee)提供了相应的α-烷基-α-叔丁氧羰基内酰胺。我们的催化方法已成功地用于白背生物碱的对映选择性全合成。通过对映选择性相转移催化烯丙基化,Dieckmann缩合和非对映选择性还原作为关键反应,从δ-戊内酰胺分12步(98%ee,43%收率)获得(+)-艾尼曲明。(-)-西比林和(+)-硝胺由(-)-异硝胺或其中间体制备。将相转移催化剂从(S,S)-NAS溴化物转换为(R,R)-NAS溴化物提供(-)-异烟酰胺(98%ee,41%产率)。(-)-Sibirine是通过(-)-异硝胺的N-乙氧羰基化反应,然后还原反应合成的(98%ee,14步,收率32%)。此外,(R)-2-苯甲酰基-2-氮杂螺[5.5]十一烷-1,7-二酮[(R)-15 ]的非对映选择性还原,然后还原性去除二苯甲基,成功得到(+)-硝胺( ee:98%,11步,收率40%)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号