Cell Reports Physical Science ( IF 8.9 ) Pub Date : 2021-01-20 , DOI: 10.1016/j.xcrp.2020.100307 Harsh Agarwal , Jacob Florian , Bryan R. Goldsmith , Nirala Singh
|
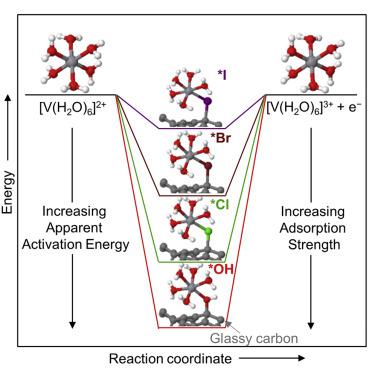
Vanadium redox flow batteries suffer from inefficiencies partly due to the kinetics of the V2+/V3+ reaction, for which lack of mechanistic understanding hinders electrolyte and electrocatalyst design to improve reaction rates. Here, we provide insights into the V2+/V3+ reaction in HClO4, H2SO4, HCl, HBr, and HI. We identify the V2+ and V3+ structures in these electrolytes using extended X-ray absorption fine structure, UV-vis, and density functional theory; this includes the hydrated structures of V2+ and V3+ in water (i.e., without anion complexation). We show that V2+/V3+ kinetics correlate with the energy of vanadium intermediate bound to carbon through a bridging anion (∗[bridge−V3+]). The anion-induced kinetic enhancement is from a decreased activation energy because of changing ∗[bridge−V3+] energy. The ∗[bridge−V3+] energy increases in the order of anion polarizability (OH− < Cl− < Br− < I−), explaining previous reports that correlate anion polarizability with the kinetics of other 3d transition metal ion redox couples.
中文翻译:

阴离子桥联对V 2+ / V 3+异质电荷转移的影响
钒氧化还原液流电池效率低下的部分原因在于V 2+ / V 3+反应的动力学,对此缺乏机械理解会阻碍电解质和电催化剂设计以提高反应速率。在这里,我们提供了有关HClO 4,H 2 SO 4,HCl,HBr和HI中的V 2+ / V 3+反应的见解。我们使用扩展的X射线吸收精细结构,UV-vis和密度泛函理论确定了这些电解质中的V 2+和V 3+结构。这包括V 2+和V 3+的水合结构在水中(即没有阴离子络合)。我们表明,V 2+ / V 3+动力学与通过桥联阴离子(* [bridge-V 3+ ])结合到碳上的钒中间体的能量相关。阴离子引起的动力学增强是由于改变* [bridge-V 3+ ]能量而使活化能降低。的* [桥-V 3+ ]在阴离子极化的顺序能量增大(OH - <氯- <溴- <I - ),说明以往的报告其归属关系阴离子极化与其他3动力学d过渡金属离子的氧化还原对。


























 京公网安备 11010802027423号
京公网安备 11010802027423号