当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Trifluoromethanesulfonyl azide as a bifunctional reagent for metal-free azidotrifluoromethylation of unactivated alkenes
Chemical Science ( IF 8.4 ) Pub Date : 2021-1-7 , DOI: 10.1039/d0sc06473d Hong-Gui Huang 1 , Weishuang Li 1 , Dayou Zhong 1 , Hu-Chong Wang 1 , Jing Zhao 1 , Wen-Bo Liu 1
Chemical Science ( IF 8.4 ) Pub Date : 2021-1-7 , DOI: 10.1039/d0sc06473d Hong-Gui Huang 1 , Weishuang Li 1 , Dayou Zhong 1 , Hu-Chong Wang 1 , Jing Zhao 1 , Wen-Bo Liu 1
Affiliation
|
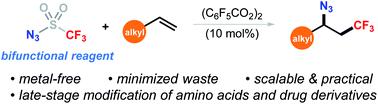
Vicinal trifluoromethyl azides have widespread applications in organic synthesis and drug development. However, their preparation is generally limited to transition-metal-catalyzed three-component reactions. We report here a simple and metal-free method that rapidly provides these building blocks from abundant alkenes and trifluoromethanesulfonyl azide (N3SO2CF3). This unprecedented two-component reaction employs readily available N3SO2CF3 as a bifunctional reagent to concurrently incorporate both CF3 and N3 groups, which avoids the use of their expensive and low atom economic precursors. A wide range of functional groups, including bio-relevant heterocycles and amino acids, were tolerated. Application of this method was further demonstrated by scale-up synthesis (5 mmol), product derivatization to CF3-containing medicinal chemistry motifs, as well as late-stage modification of natural product and drug derivatives.
中文翻译:

三氟甲磺酰叠氮化物作为双功能试剂,用于未活化烯烃的无金属叠氮三氟甲基化
邻位三氟甲基叠氮化物在有机合成和药物开发中具有广泛的应用。然而,它们的制备通常限于过渡金属催化的三组分反应。我们在这里报告了一种简单且无金属的方法,该方法可快速从丰富的烯烃和三氟甲磺酰叠氮化物(N 3 SO 2 CF 3)提供这些结构单元。这种前所未有的两组分反应采用了容易获得的N 3 SO 2 CF 3作为双功能试剂,可同时引入CF 3和N 3族避免使用它们昂贵且低原子的经济前体。宽泛的官能团,包括与生物有关的杂环和氨基酸,都可以耐受。该方法的应用通过放大合成(5 mmol),产物衍生化为含CF 3的药用化学图案以及天然产物和药物衍生物的后期修饰而得到进一步证明。
更新日期:2021-01-19
中文翻译:

三氟甲磺酰叠氮化物作为双功能试剂,用于未活化烯烃的无金属叠氮三氟甲基化
邻位三氟甲基叠氮化物在有机合成和药物开发中具有广泛的应用。然而,它们的制备通常限于过渡金属催化的三组分反应。我们在这里报告了一种简单且无金属的方法,该方法可快速从丰富的烯烃和三氟甲磺酰叠氮化物(N 3 SO 2 CF 3)提供这些结构单元。这种前所未有的两组分反应采用了容易获得的N 3 SO 2 CF 3作为双功能试剂,可同时引入CF 3和N 3族避免使用它们昂贵且低原子的经济前体。宽泛的官能团,包括与生物有关的杂环和氨基酸,都可以耐受。该方法的应用通过放大合成(5 mmol),产物衍生化为含CF 3的药用化学图案以及天然产物和药物衍生物的后期修饰而得到进一步证明。


























 京公网安备 11010802027423号
京公网安备 11010802027423号