当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
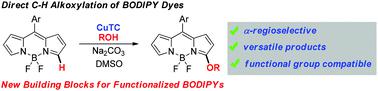
Direct C–H alkoxylation of BODIPY dyes via cation radical accelerated oxidative nucleophilic hydrogen substitution: a new route to building blocks for functionalized BODIPYs
Chemical Communications ( IF 4.9 ) Pub Date : 2021-1-8 , DOI: 10.1039/d0cc07961h Heng Li 1, 2, 3, 4, 5 , Fan Lv 1, 2, 3, 4, 5 , Xing Guo 1, 2, 3, 4, 5 , Qinghua Wu 1, 2, 3, 4, 5 , Hao Wu 1, 2, 3, 4, 5 , Bing Tang 1, 2, 3, 4, 5 , Changjiang Yu 1, 2, 3, 4, 5 , Hua Wang 1, 2, 3, 4, 5 , Lijuan Jiao 1, 2, 3, 4, 5 , Erhong Hao 1, 2, 3, 4, 5
Chemical Communications ( IF 4.9 ) Pub Date : 2021-1-8 , DOI: 10.1039/d0cc07961h Heng Li 1, 2, 3, 4, 5 , Fan Lv 1, 2, 3, 4, 5 , Xing Guo 1, 2, 3, 4, 5 , Qinghua Wu 1, 2, 3, 4, 5 , Hao Wu 1, 2, 3, 4, 5 , Bing Tang 1, 2, 3, 4, 5 , Changjiang Yu 1, 2, 3, 4, 5 , Hua Wang 1, 2, 3, 4, 5 , Lijuan Jiao 1, 2, 3, 4, 5 , Erhong Hao 1, 2, 3, 4, 5
Affiliation
|
Oxidative nucleophilic α-hydrogen substitution is a direct method for BODIPY functionalization. However, it was hampered by the low reactivity of BODIPYs toward weak nucleophiles. Herein, we develop a cation radical accelerated oxidative nucleophilic α-hydrogen substitution reaction between BODIPY dyes and a variety of alcohols. This direct C–H alkoxylation presented a wide substrate scope and high site selectivity, providing a series of α-alkoxylated BODIPYs with diverse functional groups. Moreover, a BODIPY derivative with a pyridinium ion was developed as a new mitochondria-targeting fluorescent probe with favorable photophysical properties.
中文翻译:

通过阳离子自由基加速氧化亲核氢取代对BODIPY染料进行直接C–H烷氧基化:一条为功能化BODIPYs构建基团的新途径
氧化亲核α-氢取代是BODIPY功能化的直接方法。然而,它被BODIPY对弱亲核试剂的低反应性所阻碍。在本文中,我们开发了BODIPY染料与多种醇之间的阳离子自由基加速氧化亲核α-氢取代反应。这种直接的C–H烷氧基化反应显示了广泛的底物范围和高的位点选择性,提供了一系列具有不同官能团的α-烷氧基化BODIPY。此外,具有吡啶鎓离子的BODIPY衍生物被开发为具有良好光物理性质的靶向线粒体的新型荧光探针。
更新日期:2021-01-19
中文翻译:

通过阳离子自由基加速氧化亲核氢取代对BODIPY染料进行直接C–H烷氧基化:一条为功能化BODIPYs构建基团的新途径
氧化亲核α-氢取代是BODIPY功能化的直接方法。然而,它被BODIPY对弱亲核试剂的低反应性所阻碍。在本文中,我们开发了BODIPY染料与多种醇之间的阳离子自由基加速氧化亲核α-氢取代反应。这种直接的C–H烷氧基化反应显示了广泛的底物范围和高的位点选择性,提供了一系列具有不同官能团的α-烷氧基化BODIPY。此外,具有吡啶鎓离子的BODIPY衍生物被开发为具有良好光物理性质的靶向线粒体的新型荧光探针。


























 京公网安备 11010802027423号
京公网安备 11010802027423号