Journal of Molecular Liquids ( IF 6 ) Pub Date : 2021-01-19 , DOI: 10.1016/j.molliq.2021.115413 Mustapha Alahiane , Rachid Oukhrib , Avni Berisha , Youssef Ait Albrimi , Rachid Ait Akbour , Hicham Abou Oualid , Hassan Bourzi , Ali Assabbane , Ayssar Nahlé , Mohamed Hamdani
|
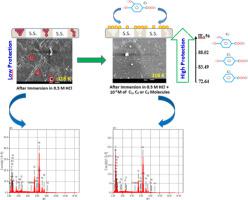
Some benzoic acid derivatives such as benzoic acid (C1), para-hydroxybenzoicacid (C2), and 3,4-dihydroxybenzoic acid (C3) were investigated as corrosion inhibitors for AISI 316 austenitic stainless steel (SS) in aggressive 0.5 M HCl solution using weight loss (WL) measurements, electrochemical measurements, and scanning electron microscopy (SEM) analysis, radial distribution function (RDF), molecular dynamics (MD). The aim of this work is to determine the inhibition efficiency based on the concentration of studied inhibitors and the temperature effect of medium. The maximum inhibition efficiency (≈ 88%) was obtained in the presence of the optimum inhibitor concentration of 1.0 × 10−2 M for C3 at 291 K. The experimental data showed that the inhibition efficiency of these inhibitors increases with the increase of their concentrations. The temperature effect on the corrosion inhibition of (SS) in 0.5 M HCl with and without inhibitors at different concentrations was investigated from 291 K to 318 K temperature range. It should be noted that each test has been carried out at least three times. Besides, the result was calculated by averaging the obtained values of each test. The activation energy and thermodynamic parameters evaluated from the weight loss measurements, suggested that the adsorption is spontaneous, exothermic, and obeyed the Villamil adsorption isotherm. SEM analyses confirmed that C3 is a good inhibitor compared with C1 and C2. Molecular dynamics simulations diagnosis for molecule/metal interaction showed that the studied organic compounds have strong interactions with Fe (110) and therefore good inhibition power for AISI 316 austenitic stainless steel (SS) against corrosion.
中文翻译:

某些苯甲酸衍生物对316不锈钢在HCl溶液中的腐蚀抑制作用的电化学,热力学和分子动力学研究
研究了一些苯甲酸衍生物,例如苯甲酸(C1),对羟基苯甲酸(C2)和3,4-二羟基苯甲酸(C3)作为AISI 316奥氏体不锈钢(SS)在腐蚀性0.5 M HCl溶液中的腐蚀抑制剂。重量损失(WL)测量,电化学测量和扫描电子显微镜(SEM)分析,径向分布函数(RDF),分子动力学(MD)。这项工作的目的是根据研究的抑制剂的浓度和介质的温度效应确定抑制效率。在最佳抑制剂浓度为1.0×10 -2的条件下,可获得最大抑制效率(≈88%)C在291 K下为M。实验数据表明,这些抑制剂的抑制效率随其浓度的增加而增加。在291 K至318 K的温度范围内,研究了在有或没有抑制剂的情况下,温度对0.5 M HCl中(SS)缓蚀性能的影响。应该注意的是,每个测试至少进行了3次。此外,通过平均每个测试的获得值来计算结果。通过失重测量评估活化能和热力学参数,表明吸附是自发的,放热的,并遵守了Villamil的吸附等温线。SEM分析证实,与C1和C2相比,C3是良好的抑制剂。

























 京公网安备 11010802027423号
京公网安备 11010802027423号