Computational and Structural Biotechnology Journal ( IF 6 ) Pub Date : 2021-01-19 , DOI: 10.1016/j.csbj.2021.01.016 Seo-Ree Choi , Jaewang Lee , Yeo-Jin Seo , Hyun Sun Kong , Minjae Kim , EonSeon Jin , Jung Ryeol Lee , Joon-Hwa Lee
|
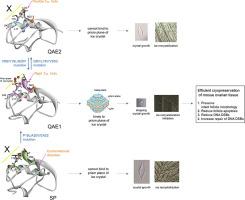
Antifreeze proteins (AFPs) can inhibit the freezing of body fluid at subzero temperatures to promote the survival of various organisms living in polar regions. Type III AFPs are categorized into three subgroups, QAE1, QAE2, and SP isoforms, based on differences in their isoelectric points. We determined the thermal hysteresis (TH), ice recrystallization inhibition (IRI), and cryopreservation activity of three isoforms of the notched-fin eelpout AFP and their mutant constructs and characterized their structural and dynamic features using NMR. The QAE1 isoform is the most active among the three classes of III AFP isoforms, and the mutants of inactive QAE2 and SP isoforms, QAE2ACT and SPACT, displayed the full TH and IRI activities with resepect to QAE1 isoform. Cryopreservation studies using mouse ovarian tissue revealed that the QAE1 isoform and the active mutants, QAE2ACT and SPACT, more effectively preserved intact follicle morphology and prevented DNA double-strand break damage more efficiently than the inactive isoforms. It was also found that all active AFPs, QAE1, QAE2ACT, and SPACT, formed unique H-bonds with the first 310 helix, an interaction that plays an important role in the formation of anchored clathrate water networks for efficient binding to the primary prism and pyramidal planes of ice crystals, which was disrupted in the inactive isoforms. Our studies provide valuable insights into the molecular mechanism of the TH and IRI activity, as well as the cryopreservation efficiency, of type III AFPs.
中文翻译:

III型抗冻蛋白的冰结合和冷冻保存活性的分子基础
抗冻蛋白(AFP)可以抑制体液在零度以下的温度下冻结,从而促进生活在极地地区的各种生物的生存。根据其等电点的差异,将III型AFP分为QAE1,QAE2和SP亚型三个亚组。我们确定了带翅鳍ee出AFP的三个同工型及其突变体的结构的热滞(TH),冰重结晶抑制(IRI)和冷冻保存活性,并使用NMR表征了其结构和动态特征。QAE1同工型是三类III AFP同工型中最活跃的,而无活性QAE2和SP同工型,QAE2 ACT和SP ACT的突变体,显示了有关QAE1亚型的完整TH和IRI活动。使用小鼠卵巢组织的冷冻保存研究表明,QAE1同工型和活性突变体QAE2 ACT和SP ACT比非活性同工型更有效地保留了完整的卵泡形态,并更有效地防止了DNA双链断裂损伤。还发现所有活跃的AFP,QAE1,QAE2 ACT和SP ACT与前3个10形成独特的H键螺旋,这种相互作用在固定的笼形水网络的形成中起着重要作用,以有效地结合到冰晶的主棱锥和金字塔平面上,而该冰晶在非活性同工型中被破坏。我们的研究为III型AFP的TH和IRI活性的分子机制以及冷冻保存效率提供了有价值的见解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号