当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Computational strategy for intrinsically disordered protein ligand design leads to the discovery of p53 transactivation domain I binding compounds that activate the p53 pathway
Chemical Science ( IF 8.4 ) Pub Date : 2020-12-28 , DOI: 10.1039/d0sc04670a Hao Ruan 1 , Chen Yu 1 , Xiaogang Niu 2, 3 , Weilin Zhang 1 , Hanzhong Liu 4 , Limin Chen 5 , Ruoyao Xiong 1 , Qi Sun 1 , Changwen Jin 2, 3 , Ying Liu 1, 4 , Luhua Lai 1, 4, 5
Chemical Science ( IF 8.4 ) Pub Date : 2020-12-28 , DOI: 10.1039/d0sc04670a Hao Ruan 1 , Chen Yu 1 , Xiaogang Niu 2, 3 , Weilin Zhang 1 , Hanzhong Liu 4 , Limin Chen 5 , Ruoyao Xiong 1 , Qi Sun 1 , Changwen Jin 2, 3 , Ying Liu 1, 4 , Luhua Lai 1, 4, 5
Affiliation
|
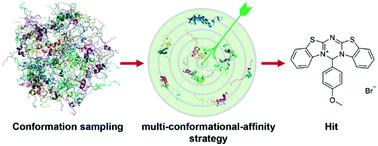
Intrinsically disordered proteins or intrinsically disordered regions (IDPs) have gained much attention in recent years due to their vital roles in biology and prevalence in various human diseases. Although IDPs are perceived as attractive therapeutic targets, rational drug design targeting IDPs remains challenging because of their conformational heterogeneity. Here, we propose a hierarchical computational strategy for IDP drug virtual screening (IDPDVS) and applied it in the discovery of p53 transactivation domain I (TAD1) binding compounds. IDPDVS starts from conformation sampling of the IDP target, then it combines stepwise conformational clustering with druggability evaluation to identify potential ligand binding pockets, followed by multiple docking screening runs and selection of compounds that can bind multi-conformations. p53 is an important tumor suppressor and restoration of its function provides an opportunity to inhibit cancer cell growth. TAD1 locates at the N-terminus of p53 and plays key roles in regulating p53 function. No compounds that directly bind to TAD1 have been reported due to its highly disordered structure. We successfully used IDPDVS to identify two compounds that bind p53 TAD1 and restore wild-type p53 function in cancer cells. Our study demonstrates that IDPDVS is an efficient strategy for IDP drug discovery and p53 TAD1 can be directly targeted by small molecules.
中文翻译:

内在无序蛋白质配体设计的计算策略导致发现激活 p53 通路的 p53 反式激活结构域 I 结合化合物
近年来,内在无序蛋白质或内在无序区域(IDP)因其在生物学中的重要作用和在各种人类疾病中的流行而受到广泛关注。尽管 IDP 被认为是有吸引力的治疗靶点,但由于其构象异质性,针对 IDP 的合理药物设计仍然具有挑战性。在这里,我们提出了一种用于 IDP 药物虚拟筛选 (IDPDVS) 的分层计算策略,并将其应用于发现 p53 反式激活结构域 I (TAD1) 结合化合物。IDPDVS 从 IDP 目标的构象采样开始,然后将逐步构象聚类与成药性评估相结合,以识别潜在的配体结合口袋,然后进行多次对接筛选运行并选择可以结合多种构象的化合物。p53是一种重要的肿瘤抑制因子,其功能的恢复提供了抑制癌细胞生长的机会。TAD1位于p53的N端,在调节p53功能中起关键作用。由于其高度无序的结构,尚未报道直接与 TAD1 结合的化合物。我们成功地使用 IDPDVS 鉴定了两种结合 p53 TAD1 并恢复癌细胞中野生型 p53 功能的化合物。我们的研究表明,IDPDVS 是一种有效的 IDP 药物发现策略,并且 p53 TAD1 可以被小分子直接靶向。由于其高度无序的结构,尚未报道直接与 TAD1 结合的化合物。我们成功地使用 IDPDVS 鉴定了两种结合 p53 TAD1 并恢复癌细胞中野生型 p53 功能的化合物。我们的研究表明,IDPDVS 是一种有效的 IDP 药物发现策略,并且 p53 TAD1 可以被小分子直接靶向。由于其高度无序的结构,尚未报道直接与 TAD1 结合的化合物。我们成功地使用 IDPDVS 鉴定了两种结合 p53 TAD1 并恢复癌细胞中野生型 p53 功能的化合物。我们的研究表明,IDPDVS 是一种有效的 IDP 药物发现策略,并且 p53 TAD1 可以被小分子直接靶向。
更新日期:2021-01-18
中文翻译:

内在无序蛋白质配体设计的计算策略导致发现激活 p53 通路的 p53 反式激活结构域 I 结合化合物
近年来,内在无序蛋白质或内在无序区域(IDP)因其在生物学中的重要作用和在各种人类疾病中的流行而受到广泛关注。尽管 IDP 被认为是有吸引力的治疗靶点,但由于其构象异质性,针对 IDP 的合理药物设计仍然具有挑战性。在这里,我们提出了一种用于 IDP 药物虚拟筛选 (IDPDVS) 的分层计算策略,并将其应用于发现 p53 反式激活结构域 I (TAD1) 结合化合物。IDPDVS 从 IDP 目标的构象采样开始,然后将逐步构象聚类与成药性评估相结合,以识别潜在的配体结合口袋,然后进行多次对接筛选运行并选择可以结合多种构象的化合物。p53是一种重要的肿瘤抑制因子,其功能的恢复提供了抑制癌细胞生长的机会。TAD1位于p53的N端,在调节p53功能中起关键作用。由于其高度无序的结构,尚未报道直接与 TAD1 结合的化合物。我们成功地使用 IDPDVS 鉴定了两种结合 p53 TAD1 并恢复癌细胞中野生型 p53 功能的化合物。我们的研究表明,IDPDVS 是一种有效的 IDP 药物发现策略,并且 p53 TAD1 可以被小分子直接靶向。由于其高度无序的结构,尚未报道直接与 TAD1 结合的化合物。我们成功地使用 IDPDVS 鉴定了两种结合 p53 TAD1 并恢复癌细胞中野生型 p53 功能的化合物。我们的研究表明,IDPDVS 是一种有效的 IDP 药物发现策略,并且 p53 TAD1 可以被小分子直接靶向。由于其高度无序的结构,尚未报道直接与 TAD1 结合的化合物。我们成功地使用 IDPDVS 鉴定了两种结合 p53 TAD1 并恢复癌细胞中野生型 p53 功能的化合物。我们的研究表明,IDPDVS 是一种有效的 IDP 药物发现策略,并且 p53 TAD1 可以被小分子直接靶向。


























 京公网安备 11010802027423号
京公网安备 11010802027423号