当前位置:
X-MOL 学术
›
Z. Anorg. Allg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydrothermal Synthesis, Crystal Structure, and Magnetism of Na2[Ir(OH)6] and its Dehydration to Na2IrO3
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.4 ) Pub Date : 2021-01-18 , DOI: 10.1002/zaac.202000454 Ralf Albrecht 1 , Hagen Poddig 1 , Jens Hunger 1 , Michael Ruck 1 , Philipp Benrath 2 , Angela Möller 3 , Thomas Doert 4
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.4 ) Pub Date : 2021-01-18 , DOI: 10.1002/zaac.202000454 Ralf Albrecht 1 , Hagen Poddig 1 , Jens Hunger 1 , Michael Ruck 1 , Philipp Benrath 2 , Angela Möller 3 , Thomas Doert 4
Affiliation
|
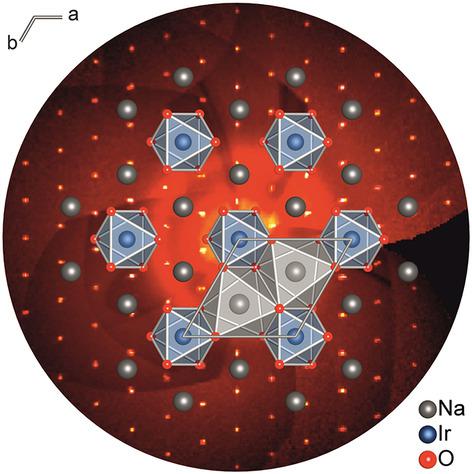
Yellow single‐crystals of Na2[Ir(OH)6] were obtained by hydrothermal synthesis in 20 m sodium hydroxide solution at 473 K within 5 hours. X‐ray diffraction on a single‐crystal revealed the trigonal space group  (no. 148) with lattice parameters a=582.76(3) pm and c=1399.30(8) pm at 100(1) K. Na2[Ir(OH)6] crystallizes in a hettotype of Mg(OH)2 (brucite) and is isostructural to Na2[Sn(OH)6]. The oxygen atoms are arranged in a hexagonal close packing, Na+ and Ir4+ ions occupy the octahedral voids in every second layer in an ordered manner, the voids of the next layers remain empty. The hydroxidometalate layer can be seen as isolated [Ir(OH)6]2− octahedra, which are embedded in a honeycomb net of alkali metal cations. Temperature‐dependent magnetic measurements revealed paramagnetic behavior with an effective moment of 1.9 μB per iridium ion. The thermal decomposition of Na2[Ir(OH)6] in air starts at about 220 °C and results in pure Na2IrO3.
(no. 148) with lattice parameters a=582.76(3) pm and c=1399.30(8) pm at 100(1) K. Na2[Ir(OH)6] crystallizes in a hettotype of Mg(OH)2 (brucite) and is isostructural to Na2[Sn(OH)6]. The oxygen atoms are arranged in a hexagonal close packing, Na+ and Ir4+ ions occupy the octahedral voids in every second layer in an ordered manner, the voids of the next layers remain empty. The hydroxidometalate layer can be seen as isolated [Ir(OH)6]2− octahedra, which are embedded in a honeycomb net of alkali metal cations. Temperature‐dependent magnetic measurements revealed paramagnetic behavior with an effective moment of 1.9 μB per iridium ion. The thermal decomposition of Na2[Ir(OH)6] in air starts at about 220 °C and results in pure Na2IrO3.
中文翻译:

Na2 [Ir(OH)6]的水热合成,晶体结构和磁性及其脱水成Na2IrO3
Na 2 [Ir(OH)6 ]的黄色单晶是通过在20 m氢氧化钠溶液中于473 K在5小时内进行水热合成而获得的 。在单晶上的X射线衍射显示在100(1)K处 晶格参数a = 582.76(3)pm和c = 1399.30(8)pm的三角空间群(148号)Na 2 [Ir(OH )6 ]结晶为Mg(OH)2(榴辉岩)的同型,并且与Na 2 [Sn(OH)6 ]同构。氧原子以六方密堆积Na +和Ir 4+排列离子以有序的方式占据了第二层中的八面体空隙,下一层的空隙保持为空。羟基金属氧酸盐层可以看作是孤立的[Ir(OH)6 ] 2-八面体,埋在碱金属阳离子的蜂巢网中。温度依赖性磁测量显示与1.9μ的有效时刻顺磁性行为乙每铱离子。Na 2 [Ir(OH)6 ]在空气中的热分解始于约220°C,并产生纯净的Na 2 IrO 3。
晶格参数a = 582.76(3)pm和c = 1399.30(8)pm的三角空间群(148号)Na 2 [Ir(OH )6 ]结晶为Mg(OH)2(榴辉岩)的同型,并且与Na 2 [Sn(OH)6 ]同构。氧原子以六方密堆积Na +和Ir 4+排列离子以有序的方式占据了第二层中的八面体空隙,下一层的空隙保持为空。羟基金属氧酸盐层可以看作是孤立的[Ir(OH)6 ] 2-八面体,埋在碱金属阳离子的蜂巢网中。温度依赖性磁测量显示与1.9μ的有效时刻顺磁性行为乙每铱离子。Na 2 [Ir(OH)6 ]在空气中的热分解始于约220°C,并产生纯净的Na 2 IrO 3。
更新日期:2021-03-23
 (no. 148) with lattice parameters a=582.76(3) pm and c=1399.30(8) pm at 100(1) K. Na2[Ir(OH)6] crystallizes in a hettotype of Mg(OH)2 (brucite) and is isostructural to Na2[Sn(OH)6]. The oxygen atoms are arranged in a hexagonal close packing, Na+ and Ir4+ ions occupy the octahedral voids in every second layer in an ordered manner, the voids of the next layers remain empty. The hydroxidometalate layer can be seen as isolated [Ir(OH)6]2− octahedra, which are embedded in a honeycomb net of alkali metal cations. Temperature‐dependent magnetic measurements revealed paramagnetic behavior with an effective moment of 1.9 μB per iridium ion. The thermal decomposition of Na2[Ir(OH)6] in air starts at about 220 °C and results in pure Na2IrO3.
(no. 148) with lattice parameters a=582.76(3) pm and c=1399.30(8) pm at 100(1) K. Na2[Ir(OH)6] crystallizes in a hettotype of Mg(OH)2 (brucite) and is isostructural to Na2[Sn(OH)6]. The oxygen atoms are arranged in a hexagonal close packing, Na+ and Ir4+ ions occupy the octahedral voids in every second layer in an ordered manner, the voids of the next layers remain empty. The hydroxidometalate layer can be seen as isolated [Ir(OH)6]2− octahedra, which are embedded in a honeycomb net of alkali metal cations. Temperature‐dependent magnetic measurements revealed paramagnetic behavior with an effective moment of 1.9 μB per iridium ion. The thermal decomposition of Na2[Ir(OH)6] in air starts at about 220 °C and results in pure Na2IrO3.
中文翻译:

Na2 [Ir(OH)6]的水热合成,晶体结构和磁性及其脱水成Na2IrO3
Na 2 [Ir(OH)6 ]的黄色单晶是通过在20 m氢氧化钠溶液中于473 K在5小时内进行水热合成而获得的 。在单晶上的X射线衍射显示在100(1)K处
 晶格参数a = 582.76(3)pm和c = 1399.30(8)pm的三角空间群(148号)Na 2 [Ir(OH )6 ]结晶为Mg(OH)2(榴辉岩)的同型,并且与Na 2 [Sn(OH)6 ]同构。氧原子以六方密堆积Na +和Ir 4+排列离子以有序的方式占据了第二层中的八面体空隙,下一层的空隙保持为空。羟基金属氧酸盐层可以看作是孤立的[Ir(OH)6 ] 2-八面体,埋在碱金属阳离子的蜂巢网中。温度依赖性磁测量显示与1.9μ的有效时刻顺磁性行为乙每铱离子。Na 2 [Ir(OH)6 ]在空气中的热分解始于约220°C,并产生纯净的Na 2 IrO 3。
晶格参数a = 582.76(3)pm和c = 1399.30(8)pm的三角空间群(148号)Na 2 [Ir(OH )6 ]结晶为Mg(OH)2(榴辉岩)的同型,并且与Na 2 [Sn(OH)6 ]同构。氧原子以六方密堆积Na +和Ir 4+排列离子以有序的方式占据了第二层中的八面体空隙,下一层的空隙保持为空。羟基金属氧酸盐层可以看作是孤立的[Ir(OH)6 ] 2-八面体,埋在碱金属阳离子的蜂巢网中。温度依赖性磁测量显示与1.9μ的有效时刻顺磁性行为乙每铱离子。Na 2 [Ir(OH)6 ]在空气中的热分解始于约220°C,并产生纯净的Na 2 IrO 3。



























 京公网安备 11010802027423号
京公网安备 11010802027423号