当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
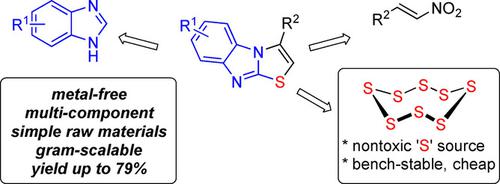
Salicylic Acid‐Promoted Three‐Component Annulation of Benzimidazoles, Aryl Nitroalkenes and Elemental Sulfur
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-01-15 , DOI: 10.1002/adsc.202001564 Ruhuai Mei 1 , Feng Xiong 1 , Chenrui Yang 1 , Jinwu Zhao 2
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-01-15 , DOI: 10.1002/adsc.202001564 Ruhuai Mei 1 , Feng Xiong 1 , Chenrui Yang 1 , Jinwu Zhao 2
Affiliation
|
Herein, a three‐component cyclization reaction of benzimidazoles, aryl nitroalkenes and elemental sulfur has been developed. Cheap and easily available salicylic acid found to be an efficient mediator for the present transformation. This protocol provides a facile access to structurally significant imidazo[2,1‐b]thiazole skeleton from simple raw materials with a range of compatible synthetically useful functionalities. Furthermore, gram‐scale preparation of this method is effective, which enables potential applications of it in broader fields of molecule synthesis. Mechanistically, a reaction cascade involving sequential aza‐Michael addition, nucleophilic sulfuration, and deaminative aromatization was proposed.
中文翻译:

水杨酸促进苯并咪唑,芳基硝基烯烃和元素硫的三组分环化
本文研究了苯并咪唑,芳基硝基烯烃和元素硫的三组分环化反应。发现便宜且容易获得的水杨酸是本转化的有效介体。该协议提供了从具有各种兼容的合成有用功能的简单原料轻松访问结构上重要的咪唑并[2,1- b ]噻唑骨架的方法。此外,这种方法的克级制备是有效的,这使其有可能在更广泛的分子合成领域中应用。从机理上讲,提出了一个反应级联反应,包括顺序的氮杂-迈克尔加成,亲核硫酸化和脱氨基芳构化。
更新日期:2021-01-15
中文翻译:

水杨酸促进苯并咪唑,芳基硝基烯烃和元素硫的三组分环化
本文研究了苯并咪唑,芳基硝基烯烃和元素硫的三组分环化反应。发现便宜且容易获得的水杨酸是本转化的有效介体。该协议提供了从具有各种兼容的合成有用功能的简单原料轻松访问结构上重要的咪唑并[2,1- b ]噻唑骨架的方法。此外,这种方法的克级制备是有效的,这使其有可能在更广泛的分子合成领域中应用。从机理上讲,提出了一个反应级联反应,包括顺序的氮杂-迈克尔加成,亲核硫酸化和脱氨基芳构化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号