当前位置:
X-MOL 学术
›
RSC Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Site-specific single point mutation by anthranilic acid in hIAPP8–37 enhances anti-amyloidogenic activity
RSC Chemical Biology Pub Date : 2021-1-15 , DOI: 10.1039/d0cb00178c Sourav Kalita 1 , Sujan Kalita 1 , Ashim Paul 1 , Manisha Shah 2 , Sachin Kumar 2 , Bhubaneswar Mandal 1
RSC Chemical Biology Pub Date : 2021-1-15 , DOI: 10.1039/d0cb00178c Sourav Kalita 1 , Sujan Kalita 1 , Ashim Paul 1 , Manisha Shah 2 , Sachin Kumar 2 , Bhubaneswar Mandal 1
Affiliation
|
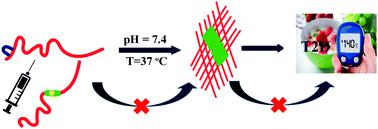
Amylin or hIAPP, together with insulin, plays a significant role in glucose metabolism. However, it undergoes β-sheet rich amyloid formation associated with pancreatic β-cell dysfunction leading to type-2 diabetes (T2D). Recent studies suggest that restricting β-sheet formation in it may halt amyloid formation, which may limit the risk for the disease. Several peptide-based inhibitors have been reported to prevent aggregation. However, most of them have limitations, including low binding efficiency, active only at higher doses, poor solubility, and proteolytic degradation. Insertion of non-coded amino acids renders proteolytically stable peptides. We incorporated a structurally rigid β-amino acid, Anthranilic acid (Ant), at different sites within the central hydrophobic region of hIAPP and developed two singly mutated hIAPP8–37 peptidomimetics. These peptidomimetics inhibited the amyloid formation of hIAPP substantially even at low concentration, as evident from in vitro ThT, CD, FT-IR, TEM, and Congo red staining birefringence results. These peptidomimetics also disrupted the preformed aggregates formed by hIAPP into non-toxic species. These β-amino acid-based peptidomimetics can be an attractive scaffold for therapeutic design towards T2D or other protein misfolding diseases.
中文翻译:

hIAPP8-37中邻氨基苯甲酸的位点特异性单点突变增强抗淀粉样蛋白生成活性
胰淀素或 hIAPP 与胰岛素一起在葡萄糖代谢中起重要作用。然而,它会形成与胰腺 β 细胞功能障碍相关的富含 β-折叠的淀粉样蛋白,导致 2 型糖尿病 (T2D)。最近的研究表明,限制其中的 β-折叠形成可能会阻止淀粉样蛋白的形成,这可能会限制该疾病的风险。据报道,几种基于肽的抑制剂可防止聚集。然而,它们中的大多数都具有局限性,包括结合效率低、仅在较高剂量下才具有活性、溶解性差和蛋白水解降解。非编码氨基酸的插入提供了蛋白水解稳定的肽。我们在 hIAPP 的中央疏水区域内的不同位点加入了结构刚性的 β-氨基酸,邻氨基苯甲酸(Ant),并开发了两个单突变的 hIAPP8-37肽模拟物。这些肽模拟物即使在低浓度下也能显着抑制 hIAPP 的淀粉样蛋白形成,这从体外ThT、CD、FT-IR、TEM 和刚果红染色双折射结果中可以明显看出。这些肽模拟物还将 hIAPP 形成的预形成聚集体破坏成无毒物种。这些基于 β-氨基酸的肽模拟物可以成为用于治疗 T2D 或其他蛋白质错误折叠疾病的有吸引力的支架。
更新日期:2021-01-15
中文翻译:

hIAPP8-37中邻氨基苯甲酸的位点特异性单点突变增强抗淀粉样蛋白生成活性
胰淀素或 hIAPP 与胰岛素一起在葡萄糖代谢中起重要作用。然而,它会形成与胰腺 β 细胞功能障碍相关的富含 β-折叠的淀粉样蛋白,导致 2 型糖尿病 (T2D)。最近的研究表明,限制其中的 β-折叠形成可能会阻止淀粉样蛋白的形成,这可能会限制该疾病的风险。据报道,几种基于肽的抑制剂可防止聚集。然而,它们中的大多数都具有局限性,包括结合效率低、仅在较高剂量下才具有活性、溶解性差和蛋白水解降解。非编码氨基酸的插入提供了蛋白水解稳定的肽。我们在 hIAPP 的中央疏水区域内的不同位点加入了结构刚性的 β-氨基酸,邻氨基苯甲酸(Ant),并开发了两个单突变的 hIAPP8-37肽模拟物。这些肽模拟物即使在低浓度下也能显着抑制 hIAPP 的淀粉样蛋白形成,这从体外ThT、CD、FT-IR、TEM 和刚果红染色双折射结果中可以明显看出。这些肽模拟物还将 hIAPP 形成的预形成聚集体破坏成无毒物种。这些基于 β-氨基酸的肽模拟物可以成为用于治疗 T2D 或其他蛋白质错误折叠疾病的有吸引力的支架。



























 京公网安备 11010802027423号
京公网安备 11010802027423号