当前位置:
X-MOL 学术
›
Microbiologyopen
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Identification of a conserved N‐terminal domain in the first module of ACV synthetases
MicrobiologyOpen ( IF 3.4 ) Pub Date : 2021-01-15 , DOI: 10.1002/mbo3.1145 Riccardo Iacovelli 1 , László Mózsik 1 , Roel A L Bovenberg 2, 3 , Arnold J M Driessen 1
MicrobiologyOpen ( IF 3.4 ) Pub Date : 2021-01-15 , DOI: 10.1002/mbo3.1145 Riccardo Iacovelli 1 , László Mózsik 1 , Roel A L Bovenberg 2, 3 , Arnold J M Driessen 1
Affiliation
|
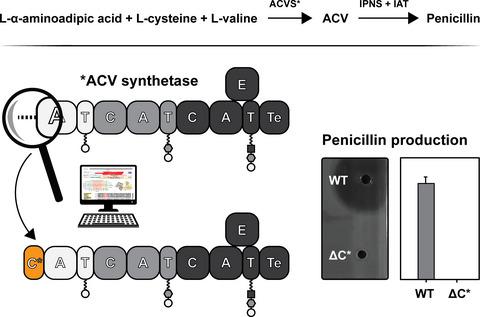
The l‐δ‐(α‐aminoadipoyl)‐l‐cysteinyl‐d‐valine synthetase (ACVS) is a trimodular nonribosomal peptide synthetase (NRPS) that provides the peptide precursor for the synthesis of β‐lactams. The enzyme has been extensively characterized in terms of tripeptide formation and substrate specificity. The first module is highly specific and is the only NRPS unit known to recruit and activate the substrate l‐α‐aminoadipic acid, which is coupled to the α‐amino group of l‐cysteine through an unusual peptide bond, involving its δ‐carboxyl group. Here we carried out an in‐depth investigation on the architecture of the first module of the ACVS enzymes from the fungus Penicillium rubens and the bacterium Nocardia lactamdurans. Bioinformatic analyses revealed the presence of a previously unidentified domain at the N‐terminus which is structurally related to condensation domains, but smaller in size. Deletion variants of both enzymes were generated to investigate the potential impact on penicillin biosynthesis in vivo and in vitro. The data indicate that the N‐terminal domain is important for catalysis.
中文翻译:

ACV 合成酶第一个模块中保守 N 端结构域的鉴定
l -δ-(α-氨基己二酰) -l-半胱氨酰-d-缬氨酸合成酶 ( ACVS ) 是一种三模块非核糖体肽合成酶 (NRPS),可为 β-内酰胺的合成提供肽前体。该酶已在三肽形成和底物特异性方面得到广泛表征。第一个模块是高度特异性的,是唯一已知募集和激活底物l -α-氨基己二酸的 NRPS 单元,该底物通过一个不寻常的肽键与l-半胱氨酸的 α-氨基偶联,包括其 δ-羧基团体。在这里,我们对来自真菌Penicillium rubens的 ACVS 酶的第一个模块的结构进行了深入研究和细菌Nocardia lactamdurans。生物信息学分析揭示了在 N 端存在一个先前未知的结构域,该结构域在结构上与缩合结构域相关,但尺寸更小。产生两种酶的缺失变体以研究对体内和体外青霉素生物合成的潜在影响。数据表明N端结构域对催化很重要。
更新日期:2021-02-16
中文翻译:

ACV 合成酶第一个模块中保守 N 端结构域的鉴定
l -δ-(α-氨基己二酰) -l-半胱氨酰-d-缬氨酸合成酶 ( ACVS ) 是一种三模块非核糖体肽合成酶 (NRPS),可为 β-内酰胺的合成提供肽前体。该酶已在三肽形成和底物特异性方面得到广泛表征。第一个模块是高度特异性的,是唯一已知募集和激活底物l -α-氨基己二酸的 NRPS 单元,该底物通过一个不寻常的肽键与l-半胱氨酸的 α-氨基偶联,包括其 δ-羧基团体。在这里,我们对来自真菌Penicillium rubens的 ACVS 酶的第一个模块的结构进行了深入研究和细菌Nocardia lactamdurans。生物信息学分析揭示了在 N 端存在一个先前未知的结构域,该结构域在结构上与缩合结构域相关,但尺寸更小。产生两种酶的缺失变体以研究对体内和体外青霉素生物合成的潜在影响。数据表明N端结构域对催化很重要。


























 京公网安备 11010802027423号
京公网安备 11010802027423号