当前位置:
X-MOL 学术
›
Chem. Eng. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Evaluation of Supercritical Technology for the Preparation of Nanomedicine: Etoricoxib Analysis
Chemical Engineering & Technology ( IF 2.1 ) Pub Date : 2021-01-15 , DOI: 10.1002/ceat.202000304 Mahboubeh Pishnamazi 1, 2 , Samyar Zabihi 3 , Sahar Jamshidian 4 , Mostafa Lashkarbolooki 5 , Fatemeh Borousan 6, 7, 8 , Azam Marjani 9, 10
Chemical Engineering & Technology ( IF 2.1 ) Pub Date : 2021-01-15 , DOI: 10.1002/ceat.202000304 Mahboubeh Pishnamazi 1, 2 , Samyar Zabihi 3 , Sahar Jamshidian 4 , Mostafa Lashkarbolooki 5 , Fatemeh Borousan 6, 7, 8 , Azam Marjani 9, 10
Affiliation
|
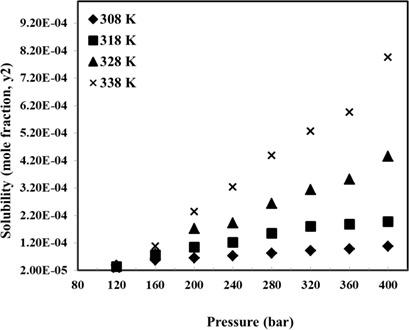
The solubility of etoricoxib in supercritical CO2 was measured using a gravimetric method in a wide range of pressures and temperatures. A pressure‐volume‐temperature (PVT) cell was designed and used. Different solubility values were obtained between 2.34 × 10−5 and 7.96 × 10−4 mol mol−1, depending on the applied pressure and temperature. Analyzing the solubility curves revealed that the pressure had a direct effect on the solubility of etoricoxib at all temperatures, while the effect of the temperature was dependent on the crossover point in the solubility curve. Indeed, before reaching the crossover point, the solubility decreased with increasing temperature, and after the crossover point, a direct relationship was observed between the solubility and the temperature. As such, the lowest solubility was obtained at 120 bar and 338 K.
中文翻译:

超临界技术制备纳米药物的评价:依托考昔分析
使用重量分析法在较宽的压力和温度范围内测量了依托考昔在超临界CO 2中的溶解度。设计并使用了压力-体积-温度(PVT)电池。在2.34×10 -5和7.96×10 -4 mol mol -1之间获得了不同的溶解度值,具体取决于施加的压力和温度。分析溶解度曲线表明,压力对依托考昔在所有温度下的溶解度都有直接影响,而温度的影响取决于溶解度曲线中的交叉点。实际上,在达到交换点之前,溶解度随温度升高而降低,而在交换点之后,观察到溶解度与温度之间存在直接关系。这样,在120巴和338K下获得最低的溶解度。
更新日期:2021-02-19
中文翻译:

超临界技术制备纳米药物的评价:依托考昔分析
使用重量分析法在较宽的压力和温度范围内测量了依托考昔在超临界CO 2中的溶解度。设计并使用了压力-体积-温度(PVT)电池。在2.34×10 -5和7.96×10 -4 mol mol -1之间获得了不同的溶解度值,具体取决于施加的压力和温度。分析溶解度曲线表明,压力对依托考昔在所有温度下的溶解度都有直接影响,而温度的影响取决于溶解度曲线中的交叉点。实际上,在达到交换点之前,溶解度随温度升高而降低,而在交换点之后,观察到溶解度与温度之间存在直接关系。这样,在120巴和338K下获得最低的溶解度。


























 京公网安备 11010802027423号
京公网安备 11010802027423号