Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Self‐Activated Cascade‐Responsive Sorafenib and USP22 shRNA Co‐Delivery System for Synergetic Hepatocellular Carcinoma Therapy
Advanced Science ( IF 15.1 ) Pub Date : 2021-01-15 , DOI: 10.1002/advs.202003042 Shengjun Xu 1, 2, 3 , Sunbin Ling 1, 2, 3 , Qiaonan Shan 2, 3 , Qianwei Ye 2, 3 , Qifan Zhan 2, 3 , Guangjiang Jiang 2, 3 , Jianyong Zhuo 2, 3 , Binhua Pan 2, 3 , Xue Wen 4 , Tingting Feng 5 , Haohao Lu 6 , Xuyong Wei 1, 2, 3 , Haiyang Xie 3 , Shusen Zheng 2, 3, 7 , Jiajia Xiang 8 , Youqing Shen 8 , Xiao Xu 1, 2, 3
Advanced Science ( IF 15.1 ) Pub Date : 2021-01-15 , DOI: 10.1002/advs.202003042 Shengjun Xu 1, 2, 3 , Sunbin Ling 1, 2, 3 , Qiaonan Shan 2, 3 , Qianwei Ye 2, 3 , Qifan Zhan 2, 3 , Guangjiang Jiang 2, 3 , Jianyong Zhuo 2, 3 , Binhua Pan 2, 3 , Xue Wen 4 , Tingting Feng 5 , Haohao Lu 6 , Xuyong Wei 1, 2, 3 , Haiyang Xie 3 , Shusen Zheng 2, 3, 7 , Jiajia Xiang 8 , Youqing Shen 8 , Xiao Xu 1, 2, 3
Affiliation
|
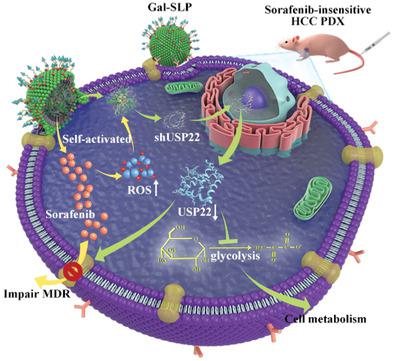
Resistance to sorafenib severely hinders its effectiveness against hepatocellular carcinoma (HCC). Cancer stemness is closely connected with resistance to sorafenib. Methods for reversing the cancer stemness remains one of the largest concerns in research and the lack of such methods obstructs current HCC therapeutics. Ubiquitin‐specific protease 22 (USP22) is reported to play a pivotal role in HCC stemness and multidrug resistance (MDR). Herein, a galactose‐decorated lipopolyplex (Gal‐SLP) is developed as an HCC‐targeting self‐activated cascade‐responsive nanoplatform to co‐delivery sorafenib and USP22 shRNA (shUSP22) for synergetic HCC therapy. Sorafenib, entrapped in the Gal‐SLPs, induced a reactive oxygen species (ROS) cascade and triggered rapid shUSP22 release. Thus, Gal‐SLPs dramatically suppressed the expression of USP22. The downregulation of USP22 suppresses multidrug resistance‐associated protein 1 (MRP1) to induce intracellular sorafenib accumulation and hampers glycolysis of HCC cells. As a result, Gal‐SLPs efficiently inhibit the viability, proliferation, and colony formation of HCC cells. A sorafenib‐insensitive patient‐derived xenograft (PDX) model is established and adopted to evaluate in vivo antitumor effect of Gal‐SLPs. Gal‐SLPs exhibit potent antitumor efficiency and biosafety. Therefore, Gal‐SLPs are expected to have great potential in the clinical treatment of HCC.
中文翻译:

用于肝细胞癌协同治疗的自激活级联响应索拉非尼和 USP22 shRNA 共传递系统
对索拉非尼的耐药性严重阻碍了其对抗肝细胞癌(HCC)的有效性。癌症干细胞性与索拉非尼耐药性密切相关。逆转癌症干性的方法仍然是研究中最受关注的问题之一,缺乏此类方法阻碍了当前的 HCC 治疗。据报道,泛素特异性蛋白酶 22 (USP22) 在 HCC 干细胞性和多药耐药性 (MDR) 中发挥着关键作用。在此,开发了一种半乳糖修饰的脂多聚复合物(Gal-SLP)作为一种针对 HCC 的自激活级联响应纳米平台,以共同递送索拉非尼和 USP22 shRNA(shUSP22)进行协同 HCC 治疗。包埋在 Gal-SLP 中的索拉非尼诱导活性氧 (ROS) 级联反应并引发 shUSP22 快速释放。因此,Gal-SLP 显着抑制了 USP22 的表达。USP22 的下调会抑制多药耐药相关蛋白 1 (MRP1),从而诱导细胞内索拉非尼积累并阻碍 HCC 细胞的糖酵解。因此,Gal-SLP 有效抑制 HCC 细胞的活力、增殖和集落形成。建立了索拉非尼不敏感的患者来源的异种移植(PDX)模型,并采用该模型来评估 Gal-SLP 的体内抗肿瘤作用。Gal-SLPs 表现出有效的抗肿瘤效率和生物安全性。因此,Gal-SLPs有望在HCC的临床治疗中具有巨大潜力。
更新日期:2021-03-03
中文翻译:

用于肝细胞癌协同治疗的自激活级联响应索拉非尼和 USP22 shRNA 共传递系统
对索拉非尼的耐药性严重阻碍了其对抗肝细胞癌(HCC)的有效性。癌症干细胞性与索拉非尼耐药性密切相关。逆转癌症干性的方法仍然是研究中最受关注的问题之一,缺乏此类方法阻碍了当前的 HCC 治疗。据报道,泛素特异性蛋白酶 22 (USP22) 在 HCC 干细胞性和多药耐药性 (MDR) 中发挥着关键作用。在此,开发了一种半乳糖修饰的脂多聚复合物(Gal-SLP)作为一种针对 HCC 的自激活级联响应纳米平台,以共同递送索拉非尼和 USP22 shRNA(shUSP22)进行协同 HCC 治疗。包埋在 Gal-SLP 中的索拉非尼诱导活性氧 (ROS) 级联反应并引发 shUSP22 快速释放。因此,Gal-SLP 显着抑制了 USP22 的表达。USP22 的下调会抑制多药耐药相关蛋白 1 (MRP1),从而诱导细胞内索拉非尼积累并阻碍 HCC 细胞的糖酵解。因此,Gal-SLP 有效抑制 HCC 细胞的活力、增殖和集落形成。建立了索拉非尼不敏感的患者来源的异种移植(PDX)模型,并采用该模型来评估 Gal-SLP 的体内抗肿瘤作用。Gal-SLPs 表现出有效的抗肿瘤效率和生物安全性。因此,Gal-SLPs有望在HCC的临床治疗中具有巨大潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号