当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of polycyclic naphthols and naphthalenes via tandem Ti(Oi-Pr)4-promoted photoenolization Diels–Alder reaction and aromatization
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2021-1-4 , DOI: 10.1039/d0qo01346c Xiao-Long Lu 1, 2, 3, 4, 5 , Baochao Yang 1, 2, 3, 4, 5 , Haibing He 3, 4, 5, 6 , Shuanhu Gao 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2021-1-4 , DOI: 10.1039/d0qo01346c Xiao-Long Lu 1, 2, 3, 4, 5 , Baochao Yang 1, 2, 3, 4, 5 , Haibing He 3, 4, 5, 6 , Shuanhu Gao 1, 2, 3, 4, 5
Affiliation
|
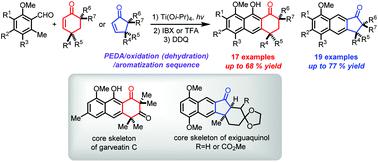
An efficient approach to naphthol and naphthalene scaffolds has been developed using a sequence involving tandem Ti(Oi-Pr)4-promoted photoenolization Diels–Alder (PEDA) and aromatization reactions. Starting with photoenolizable ortho-tolualdehyde derived dienes, the sequence using sterically hindered cyclohexenone and cyclopentenone dienophiles construct naphthol- and naphthalene-fused polycyclic products, respectively. The strategy was successfully utilized to synthesize the core skeletons of the bioactive marine natural products garveatin C and exiguaquinol.
中文翻译:

串联Ti(Oi-Pr)4促进的光致烯化反应Diels-Alder反应和芳构化反应合成多环萘和萘
已经开发出一种有效的方法来制备萘酚和萘支架,该方法涉及串联Ti(O i -Pr )4促进的光致脱色Diels-Alder(PEDA)和芳构化反应的序列。从可光烯化的邻甲苯甲醛衍生的二烯开始,使用空间位阻的环己烯酮和环戊烯酮二烯亲和物的序列分别构建了萘酚和萘稠合的多环产物。该策略已成功用于合成具有生物活性的海洋天然产物garveatin C和exiguaquinol的核心骨架。
更新日期:2021-01-14
中文翻译:

串联Ti(Oi-Pr)4促进的光致烯化反应Diels-Alder反应和芳构化反应合成多环萘和萘
已经开发出一种有效的方法来制备萘酚和萘支架,该方法涉及串联Ti(O i -Pr )4促进的光致脱色Diels-Alder(PEDA)和芳构化反应的序列。从可光烯化的邻甲苯甲醛衍生的二烯开始,使用空间位阻的环己烯酮和环戊烯酮二烯亲和物的序列分别构建了萘酚和萘稠合的多环产物。该策略已成功用于合成具有生物活性的海洋天然产物garveatin C和exiguaquinol的核心骨架。


























 京公网安备 11010802027423号
京公网安备 11010802027423号