当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Bioconjugate Supramolecular Pd2+ Metallacages Penetrate the Blood Brain Barrier In Vitro and In Vivo
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2021-01-13 , DOI: 10.1021/acs.bioconjchem.0c00659 Ben Woods 1 , Rúben D M Silva 2 , Claudia Schmidt 3 , Darren Wragg 3 , Marco Cavaco 4 , Vera Neves 4 , Vera F C Ferreira 2 , Lurdes Gano 2, 5 , Tânia S Morais 6 , Filipa Mendes 2, 5 , João D G Correia 2, 5 , Angela Casini 3
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2021-01-13 , DOI: 10.1021/acs.bioconjchem.0c00659 Ben Woods 1 , Rúben D M Silva 2 , Claudia Schmidt 3 , Darren Wragg 3 , Marco Cavaco 4 , Vera Neves 4 , Vera F C Ferreira 2 , Lurdes Gano 2, 5 , Tânia S Morais 6 , Filipa Mendes 2, 5 , João D G Correia 2, 5 , Angela Casini 3
Affiliation
|
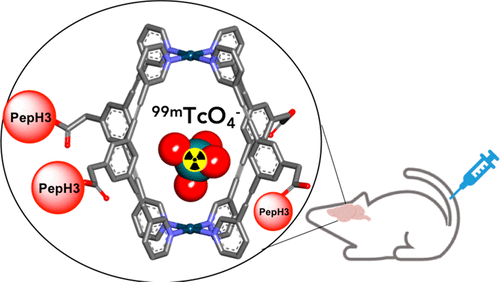
The biomedical application of discrete supramolecular metal-based structures, specifically self-assembled metallacages, is still an emergent field of study. Capitalizing on the knowledge gained in recent years on the development of 3-dimensional (3D) metallacages as novel drug delivery systems and theranostic agents, we explore here the possibility to target [Pd2L4]4+ cages (L = 3,5-bis(3-ethynylpyridine)phenyl ligand) to the brain. In detail, a new water-soluble homoleptic cage (CPepH3) tethered to a blood brain barrier (BBB)-translocating peptide was synthesized by a combination of solid-phase peptide synthesis (SPPS) and self-assembly procedures. The cage translocation efficacy was assessed by inductively coupled mass spectrometry (ICP-MS) in a BBB cellular model in vitro. Biodistribution studies of the radiolabeled cage [[99mTcO4]− ⊂ CPepH3] in the CD1 mice model demonstrate its brain penetration properties in vivo. Further DFT studies were conducted to model the structure of the [[99mTcO4]− ⊂ cage] complex. Moreover, the encapsulation capabilities and stability of the cage were investigated using the [ReO4]− anion, the “cold” analogue of [99mTcO4]−, by 1H NMR spectroscopy. Overall, our study constitutes another proof-of-concept of the unique potential of supramolecular coordination complexes for modifying the physiochemical and biodistribution properties of diagnostic species.
中文翻译:

生物共轭物超分子 Pd2+ Metallacages 在体外和体内穿透血脑屏障
离散超分子金属基结构的生物医学应用,特别是自组装金属笼,仍然是一个新兴的研究领域。利用近年来在开发 3D (3D) 金属笼作为新型药物递送系统和治疗诊断剂方面获得的知识,我们在此探索靶向 [Pd 2 L 4 ] 4+笼(L = 3,5)的可能性-双(3-乙炔基吡啶)苯基配体)到大脑。详细地说,一种新的水溶性均质笼(C PepH3) 通过固相肽合成 (SPPS) 和自组装程序的组合合成血脑屏障 (BBB) 易位肽。通过电感耦合质谱 (ICP-MS)在体外BBB 细胞模型中评估笼易位功效。放射性标记笼 [[ 99m TcO 4 ] - ⊂ C PepH3 ] 在 CD1 小鼠模型中的生物分布研究证明了其在体内的脑渗透特性。进行了进一步的 DFT 研究以模拟 [[ 99m TcO 4 ] - 的结构⊂笼】复。此外,使用[ReO 4 ] -阴离子([ 99m TcO 4 ] -的“冷”类似物)通过1 H NMR 光谱研究了笼的封装能力和稳定性。总体而言,我们的研究构成了超分子配位复合物在改变诊断物种的理化和生物分布特性方面的独特潜力的另一个概念证明。
更新日期:2021-01-13
中文翻译:

生物共轭物超分子 Pd2+ Metallacages 在体外和体内穿透血脑屏障
离散超分子金属基结构的生物医学应用,特别是自组装金属笼,仍然是一个新兴的研究领域。利用近年来在开发 3D (3D) 金属笼作为新型药物递送系统和治疗诊断剂方面获得的知识,我们在此探索靶向 [Pd 2 L 4 ] 4+笼(L = 3,5)的可能性-双(3-乙炔基吡啶)苯基配体)到大脑。详细地说,一种新的水溶性均质笼(C PepH3) 通过固相肽合成 (SPPS) 和自组装程序的组合合成血脑屏障 (BBB) 易位肽。通过电感耦合质谱 (ICP-MS)在体外BBB 细胞模型中评估笼易位功效。放射性标记笼 [[ 99m TcO 4 ] - ⊂ C PepH3 ] 在 CD1 小鼠模型中的生物分布研究证明了其在体内的脑渗透特性。进行了进一步的 DFT 研究以模拟 [[ 99m TcO 4 ] - 的结构⊂笼】复。此外,使用[ReO 4 ] -阴离子([ 99m TcO 4 ] -的“冷”类似物)通过1 H NMR 光谱研究了笼的封装能力和稳定性。总体而言,我们的研究构成了超分子配位复合物在改变诊断物种的理化和生物分布特性方面的独特潜力的另一个概念证明。


























 京公网安备 11010802027423号
京公网安备 11010802027423号