当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
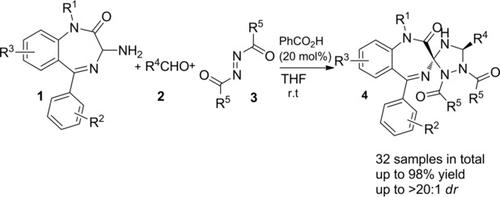
Formal [3+2] Cycloaddition Between in situ Formed 1,4‐Benzodiazepin‐2‐One‐Based Azomethine Ylides and Azodicarboxylic Acid Derivatives: Diastereoselective Synthesis of Spiro‐1,4‐Benzodiazepin‐2‐Ones
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-01-13 , DOI: 10.1002/adsc.202001486 Xiao‐Zu Fan 1 , Hui‐Hui Wu 1 , Zhe Tang 1 , Heng Zhang 1 , Lu‐Yu Cai 1 , Xiao‐Fan Bi 1 , Hong‐Wu Zhao 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-01-13 , DOI: 10.1002/adsc.202001486 Xiao‐Zu Fan 1 , Hui‐Hui Wu 1 , Zhe Tang 1 , Heng Zhang 1 , Lu‐Yu Cai 1 , Xiao‐Fan Bi 1 , Hong‐Wu Zhao 1
Affiliation
|
In the presence of PhCO2H (20 mol%), the formal [3+2] cycloaddition between in situ formed 1,4‐benzodiazepin‐2‐one‐based azomethine ylides and azodicarboxylic acid derivatives proceeded readily, thus leading to the formation of trans‐configured spiro‐1,4‐benzodiazepin‐2‐ones in up to 98% chemical yield with >20:1 dr. The relative configuration of the title compounds was unambiguously determined by means of X‐ray single crystal structure analysis. The reaction mechanism was hypothesized to account for the diastereoselective formation of the isolated spiro‐1,4‐benzodiazepin‐2‐ones.
中文翻译:

原位形成的1,4-苯并二氮杂-2-2-1基甲氧亚胺叶立德化合物和偶氮二羧酸衍生物之间的正式[3 + 2]环加成反应:螺-1,4-苯并二氮杂-2-2的非对映选择性合成
在存在PhCO 2 H(20 mol%)的情况下,原位形成的1,4-苯并二氮杂-2-2-酮基偶氮甲亚胺与偶氮二羧酸衍生物之间的正式[3 + 2]环加成反应很容易进行,从而导致形成的反式-型螺- 1,4-苯并二氮杂-2-酮在高达98%的化学产率> 20:1个 博士。标题化合物的相对构型是通过X射线单晶结构分析明确确定的。假设反应机理是考虑到了孤立的螺-1,4-苯并二氮杂-2--1-酮的非对映选择性形成。
更新日期:2021-03-03
中文翻译:

原位形成的1,4-苯并二氮杂-2-2-1基甲氧亚胺叶立德化合物和偶氮二羧酸衍生物之间的正式[3 + 2]环加成反应:螺-1,4-苯并二氮杂-2-2的非对映选择性合成
在存在PhCO 2 H(20 mol%)的情况下,原位形成的1,4-苯并二氮杂-2-2-酮基偶氮甲亚胺与偶氮二羧酸衍生物之间的正式[3 + 2]环加成反应很容易进行,从而导致形成的反式-型螺- 1,4-苯并二氮杂-2-酮在高达98%的化学产率> 20:1个 博士。标题化合物的相对构型是通过X射线单晶结构分析明确确定的。假设反应机理是考虑到了孤立的螺-1,4-苯并二氮杂-2--1-酮的非对映选择性形成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号